Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 01.06.2022

Peptides represent a powerful class of drugs due to their low toxicity, good tolerability, high selectivity, and high potency. However, the identification and validation of novel peptide drug leads is time- and cost-consuming as it requires high-throughput manufacturing of peptide libraries (N > 24) with solid-phase peptide synthesis (SPPS). Here, the absence of by-products and other impurities is vital for reliable assay results. However, because individual synthesis optimizations are cumbersome and parallel purification with high-pressure liquid chromatography (HPLC) is not feasible, developers often waste enormous time and resources manufacturing and purifying their most promising lead compounds, or low-purity crude peptides are used which might lead to false-negative or false-positive results.
Belyntic overcomes this bottleneck by customization of the Peptide Easy Clean (PEC) purification technology to a 96 well-plate format.
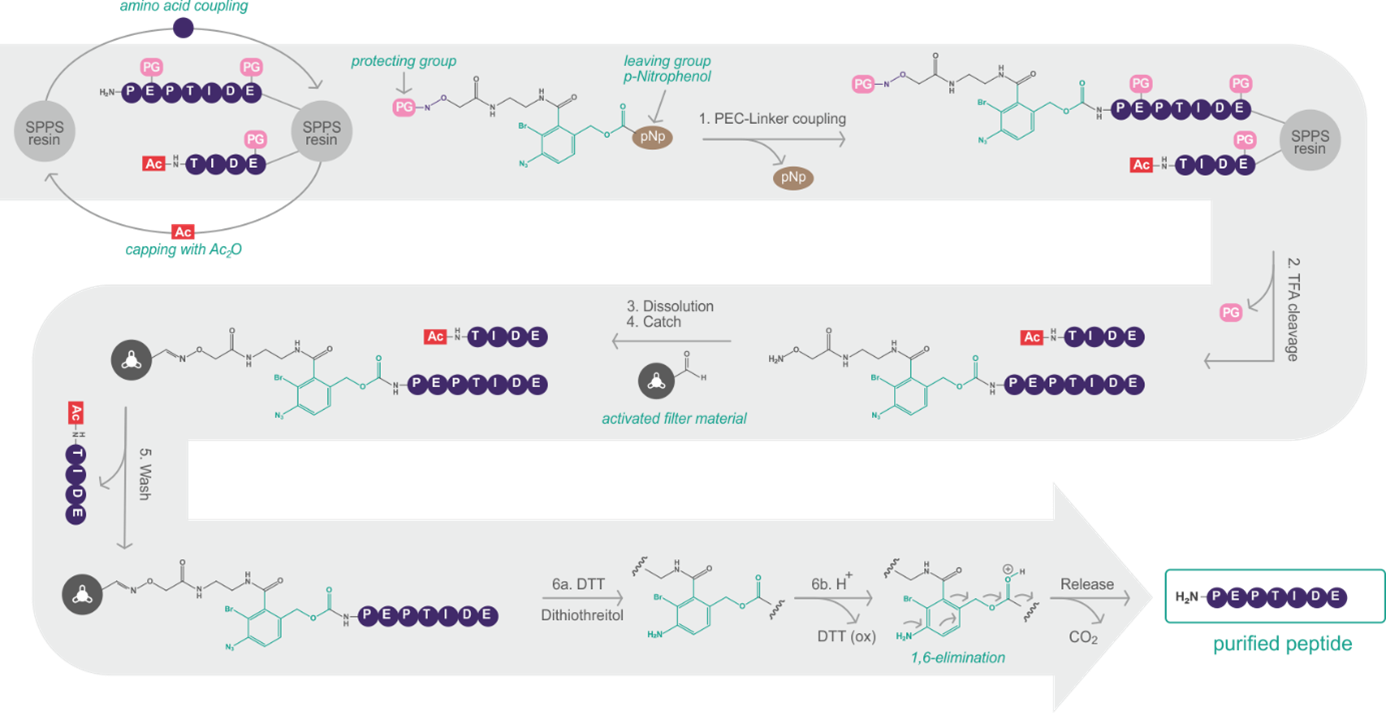
Schematic illustration of the PEC purification.
We are focusing on efficacy and are aiming to shorten the development and production times for the ideal therapeutic or diagnostic agent, regardless of its complexity. Belyntic’s PEC technology can support the customer’s activities along the entire value chain of pharmaceutical development and production – from delivering purified peptide libraries for drug discovery, to sophisticated modifications for lead optimization, to economically and ecologically efficient large-scale manufacturing.
The PEC technology allows:
In addition, Belyntic’s experienced technical team in Germany can perform feasibility and optimization studies for specific peptide projects. Get in contact for more information, we are pleased to support you and your research!
References:
Peptide Impurities in Commercial Synthetic Peptides and Their Implications for Vaccine Trail Assessment; J. R. Currier, L. M. Galley, H. Wenschuh, V. Morafo, S. Ratto-Kim, C. M. Gray, L. Maboko, M. Hoelscher, M. A. Marovich, J. H. Cox; Clin. Vaccine Immunol. 2008, 15, 267-276. https://doi.org/10.1128/CVI.00284-07
The impact of impurities in synthetic peptides on the outcome of t-cell stimulation assays; J. W. K. de Beukelaar, J. W Gratama, P. A. Smitt, G M. G. M. Verjans, J. Kraan, T. M. Luider, P. C. Burgers; Rapid Commun. Mass Spectrom. 2007, 21, 1282-1288. https://doi.org/10.1002/rcm.2958
T Cells Recognizing a Peptide Contaminant Undetectable by Mass Spectrometry; V. Brezar, S. Culina, T. Østerbye, F. Guillonneau, G. Chiappetta, Y. Verdier, J. Vinh, F. S. Wong, S. Buus, R. Mallone, Plos One 2011, 6, e28866. https://doi.org/10.1371/journal.pone.0028866