Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 30.09.2016
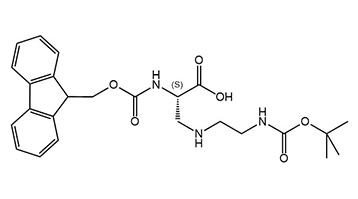
Unnatural amino acids with several positive charges are useful tools for expanding the chemical space in peptide design. They can be employed as mimics of natural amino acids in peptides, or as building blocks for combinatorial chemistry. The polarity of cationic amino acids can improve cellular uptake of peptides, since a high number of positive charges in peptides seem to correlate with high uptake efficiency in human cells. Consequently, membrane-interacting peptides and antimicrobial peptides usually bear several basic residues. Furthermore, many biologically active natural products contain non-canonical cationic amino acids.
Triamino acids are also interesting for the preparation of dendrimers with a higher degree of amine functionalization than lysine dendrimers.
We now offer Fmoc-L-Dap(2-Boc-aminoethyl)-OH as our first triamino acid building block, and are currently working on expanding our portfolio with further analogous triamino acids.