Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 27.01.2016
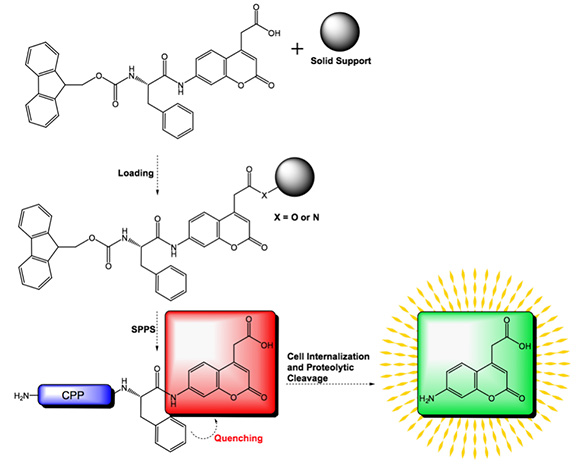
The unnatural amino acid Aca (7-amino-coumarin-4-acetic acid) is a coumarin derivative and thus exhibits fluorescence. When incorporated into a peptide C-terminally of phenylalanine, Aca is a useful reporter group for the successful internalization of CPPs. The phenyl moiety of Phe quenches the fluorescence of Aca. Internalization of the CPP containing Phe-Aca leads to proteolytic cleavage of the Phe-Aca bond and thus to fluorescence.
However, the peptide bond formation between Phe and Aca is considered to be a difficult coupling and often leads to low coupling yields. For your convenience, Iris Biotech offers Fmoc Phe-Aca as building block suitable for SPPS. This pseudodipeptide can be coupled to the resin of your choice and subsequently elongated to prepare your target cell penetrating peptides.
References: