Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 02.06.2021
Peptides and proteins are widely utilized in different fields of research such as drug development, the generation of functional biomaterials, or as probes to investigate biological processes and functions.
Despite advances in peptide chemistry, the synthesis of large proteins remains challenging and often requires the ligation of several separately synthesized peptide fragments which often suffer from insolubility and aggregation. Besides using special solvent mixtures and additives such as detergents, the addition of solubilizing tags (e.g., short charged peptide stretches) revealed as effective strategy to overcome those limitations. Especially the reversible attachment of solubilizing tags, which can be removed after protein assembly in a traceless manner are highly aimed for as otherwise the residual tag might impact the folding and function of the final target.
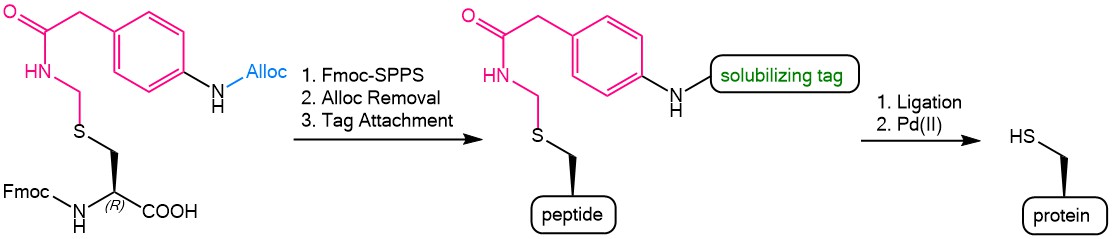
Iris Biotech offers an Fmoc protected para allyloxycarbonyl (Alloc) amino-functionalized phenylactamidomethyl (Phacm) Cysteine derivative = Fmoc-L-Cys(Aapam)-OH (FAA5150). The following scheme presents the possibilities offered by this versatile building block. The Alloc-Phacm Cys can easily be introduced in peptides during Fmoc-solid phase peptide synthesis. The Alloc group can then be removed by using tetrakis(triphenylphosphine)palladium(0) [Pd(PPh3)4] in the presence of phenylsilane with the Phacm group being completely stable under these conditions. After Alloc-deprotection, further groups can be coupled to the remaining free amine of Phacm, e.g. a solubilizing tag. The fully synthesized peptide can be cleaved from the resin by using TFA and the solubilized peptide fragments can be assembled by ligation. Finally, the Phacm-linked solubilizing tag can easily be removed in solution by treatment with PdCl2 to yield the fully unprotected Cysteine side chain.
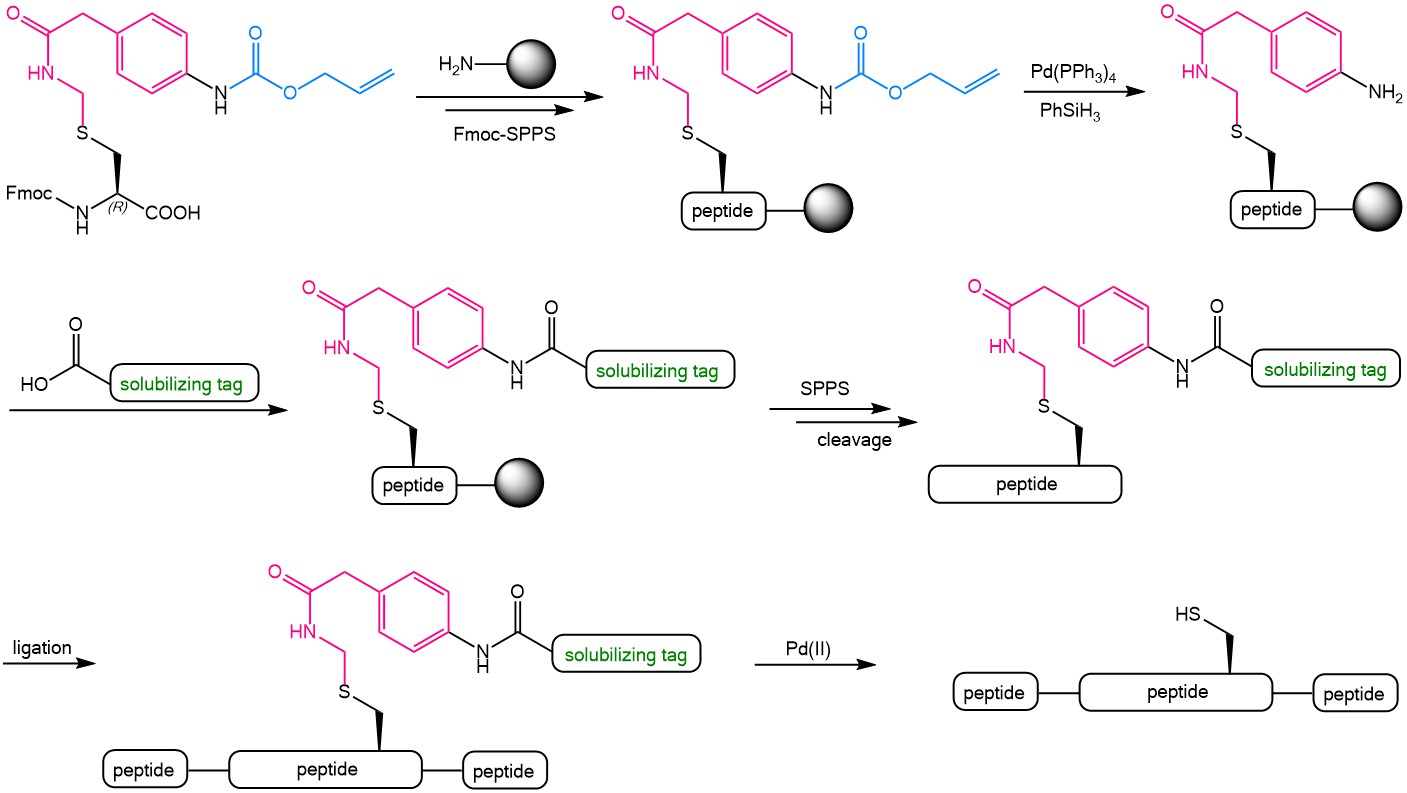
The use of the Alloc-Phacm (Aapam) linker during Fmoc-SPPS and protein synthesis.
This commercially available Alloc-protected Phacm linker will significantly facilitate the synthesis of challenging insoluble proteins from multiple fragments. As the resulting free Cysteine can be converted to Alanine through desulfurization, the Alloc-Phacm linker can be used as side chain modification to incorporate solubilizing tags at the position of Cysteine but also of Alanine.
Reference:
Palladium Mediated Rapid Deprotection of N-Terminal Cysteine under Native Chemical Ligation Conditions for the Efficient Preparation of Synthetically Challenging Proteins. Muhammad Jbara, Suman Kumar Maity, Mallikanti Seenaiah, Ashraf Brik; J. Am. Chem. Soc. 2016; 138(15): 5069-5075. https://doi.org/10.1021/jacs.5b13580.
Palladium-assisted removal of a solubilizing tag from a Cys Side Chain to Facilitate Peptide and Protein Synthesis; S. K. Maity, G. Mann, M Jbara, S. Laps, G. Kamnesky, A. Brik; Org. Lett. 2016; 18(12): 3026-3029. https://doi.org/10.1021/acs.orglett.6b01442.
Total Chemical Synthesis of Proteins; A. Brik, P. Dawson, L. Liu; 2021; Wiley-VCH, Weinheim; 2001. ISBN: 978-3-527-34660-8.