Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
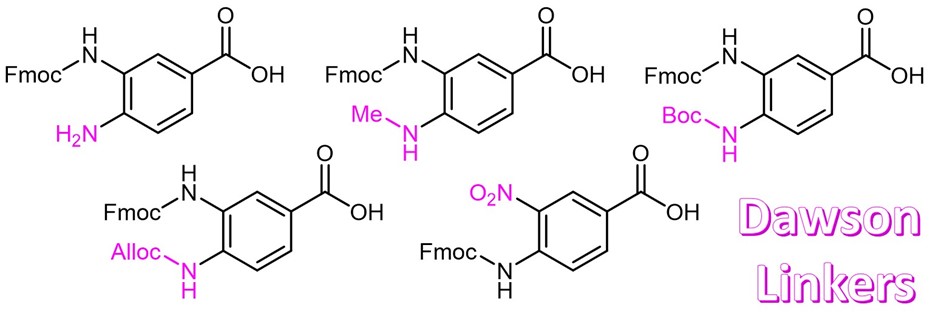
Continue to Iris Biotech GmbHSend request to US distributorChemischer Name: 3-[(9-Fluorenylmethyloxycarbonyl)amino]-4-(methylamino)benzoic acid // Synonyme: 3-(Fmoc-amino)-4-(methylamino)benzoic acid, Dawson-Linker
Startet von 86,00 €
MeDbz is a novel linker for the synthesis of peptide thioesters by Fmoc SPPS . After Fmoc removal the resin is being acylated with the first amino acid and then peptide synthesis is carried out. The 4-N-methyl group suppresses the formation of side products on the para-amino moiety. Following chain assembly the resin is being activated by treatment with p-nitrophenyl chloroformate. TFA cleaves the fully deprotected peptide benzimidazolinone which can be converted to a thioester with aryl thiol or used directly in native chemical ligation.
Fmoc-MeDbz is used as a linker in peptide synthesis. It can be cyclysed into a N-acyl-N-methylacylurea using Nitrophenyl chloroformate.
Solid-Phase Synthesis of Head to Side-Chain Tyr-Cyclodepsipeptides Through a Cyclative Cleavage From Fmoc-MeDbz/MeNbz-resins; G. A. Acosta, L. Murray, M. Royo, B. G. de la Torre and F. Albericio; Frontiers in Chemistry 2020; 8. https://doi.org/10.3389/fchem.2020.00298
A Reversible Protection Strategy To Improve Fmoc-SPPS of Peptide Thioesters by the N-Acylurea Approach; Santosh K. Mahto, Cecil J. Howard, John C. Shimko, and Jennifer J. Ottesen;
ChemBioChem 2011; 12: 2488-2494. DOI: 10.1002/cbic.201100472.
An efficient Fmoc-SPPS approach for the generation of thioester peptide precursors for use in native chemical ligation; Blanco-Canosa, J. B. & Dawson, P. E.; Angew. Chem. Int. Ed.
2008; 47: 6851-6855.
Z. Harpaz, et al.; ChemBioChem 2010; 11: 1232.
B. L. Pentelute, et al., Chem. Biol. 2010; 5: 359.
T. K. Tiefenbrunn, et al.; Pept. Sci. 2010; 94: 405.
Blanco-Canosa, Juan B.; Nardone, Brunello; Albericio, Fernando; Dawson, Philip E. Journal of the American Chemical Society (2015), 137(22), 7197-7209. DOI:10.1021/jacs.5b03504;
Jbara, Muhammad; Maity, Suman Kumar; Seenaiah, Mallikanti; Brik, Ashraf Journal of the American Chemical Society (2016), 138(15), 5069-5075. DOI:10.1021/jacs.5b13580;
Wever, Walter J.; Bogart, Jonathan W.; Bowers, Albert A. Journal of the American Chemical Society (2016), 138(41), 13461-13464. DOI:10.1021/jacs.6b05389; Abdel Monaim, Shimaa A. H.; Acosta, Gerardo A.; Royo, Miriam; El-Faham, Ayman; de la Torre, Beatriz G.; Albericio, Fernando Tetrahedron Letters (2018), 59(18), 1779-1782. DOI:10.1016/j.tetlet.2018.03.084;
Tsuda, Shugo; Mochizuki, Masayoshi; Ishiba, Hiroyuki; Yoshizawa-Kumagaye, Kumiko; Nishio, Hideki; Oishi, Shinya; Yoshiya, Taku Angewandte Chemie, International Edition (2018), 57(8), 2105-2109. DOI:10.1002/anie.201711546.
Sie benötigen größere Mengen für Ihre Entwicklung oder Produktion?
Bitte senden Sie mir mehr Informationen über