Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
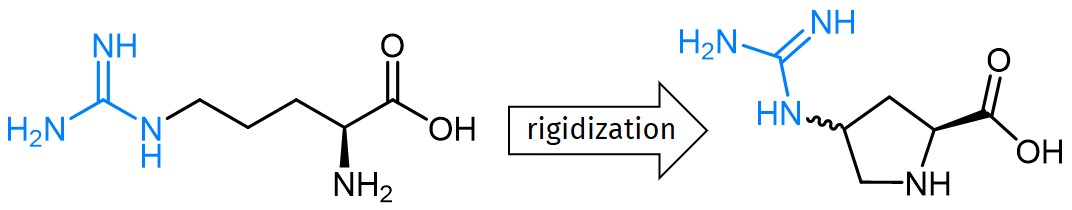
Continue to Iris Biotech GmbHSend request to US distributorChemischer Name: (2S,4S)-1-(9-Fluorenylmethyloxycarbonyl)-4-(3-(2,2,4,6,7-pentamethyl-2,3-dihydrobenzofuran-5-ylsulfonyl)guanidino)pyrrolidine-2-carboxylic acid // Synonyme: (2S,4S)-Fmoc-4-(N'-Pbf-guanidino)-pyrrolidine-2-carboxylic acid,(2S,4S)-1-(((9H-fluoren-9-yl)methoxy)carbonyl)-4-(3-(2,2,4,6,7-pentamethyl-2,3-dihydrobenzofuran-5-ylsulfonyl)guanidino)pyrrolidine-2- carboxylic acid Fmoc-Pro(4-guanidino-Pbf)-OH(2S,4S
Startet von 275,00 €
Structural Development of Cell-Penetrating Peptides Containing Cationic Proline Derivatives; H. Kobayashi, T. Misawa, M. Oba, N. Hirata, Y. Kanda, M. Tanaka, K. Matsuno, Y. Demizu; Chem. Pharm. Bulletin 2018; 66(5): 575-580. https://doi.org/10.1248/cpb.c18-00079.
Effect of Preorganized Charge-Display on the Cell-Penetrating Properties of Cationic Peptides; Y. A. Nagel, P. S. Raschle, H. Wennemers; Angew. Chem. Int. Ed. 2017; 56(1): 122-126. https://doi.org/10.1002/anie.201607649.
Plasmid DNA delivery by arginine-rich cell-penetrating peptides containing unnatural amino acids; T. Kato, H. Yamashita, T. Misawa, K. Nishida, M. Kurihara, M. Tanaka, Y. Demizu, M. Oba; Bioorg. Med. Chem. 2016; 24(12): 2681-2687. https://doi.org/10.1016/j.bmc.2016.04.031.
Substitution of Arginine with Proline and Proline Derivatives in Melanocyte-Stimulating Hormones Leads to Selectivity for Human Melanocortin 4 Receptor; H. Qu, M. Cai, A. V. Mayorov, P. Grieco, M. Zingsheim, D. Trivedi, V. J. Hruby; J. Med. Chem. 2009; 52(12): 3627-3635. https://doi.org/10.1021/jm801300c.
Rational Design of a-Conotoxin Analogues Targeting a7 Nicotinic Acetylcholine Receptors; C. Armishaw, A. A. Jensen, T. Balle, R. J. Clark, K. Harpsøe, C. Skonberg, T. Liljefors, K. Strømgaard; J. Biol. Chem. 2009; 284(14): 9498-9512. https://doi.org/10.1074/jbc.M806136200.
Synthesis of Proteins Containing Modified Arginine Residues; A. K. Choudhury, S. Y. Golovine, L. M. Dedkova, S. M. Hecht; Biochem. 2007; 46(13): 406-4076. https://doi.org/10.1021/bi062042r.
Practical and Efficient Synthesis of Orthogonally Protected Constrained 4-Guanidinoprolines; M. Tamaki, G. Han, V. J. Hruby; J. Org. Chem. 2001; 66(3): 1038-1042. https://doi.org/10.1021/jo005626m.
Synthesis of Alanine and Proline Amino Acids with Amino or Guanidinium Substitution on the Side Chain; Z. Zhang, A. V. Aerschot, C. Hendrix, R. Busson, F. David, P. Sandra, P. Herdewijn; Tetrahedron 2000; 56(16): 2513-2522. https://doi.org/10.1016/S0040-4020(00)00123-X.
Conformationally restricted arginine analogs; T. R. Webb, C. Eigenbrot; J. Org. Chem. 1991; 56(9): 3009-3016. https://doi.org/10.1021/jo00009a016.
Sie benötigen größere Mengen für Ihre Entwicklung oder Produktion?
Bitte senden Sie mir mehr Informationen über