Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorα-Methyl and alkyl-amino acids can be produced by a number of platforms, where the (2S,4S)-4-methyl-2-phenyloxazolidin-5-one scaffold is one of the most popular one. We optimize published procedures and have own proprietary ones, which enable us to supply all kinds of protected α-methyl amino acids.
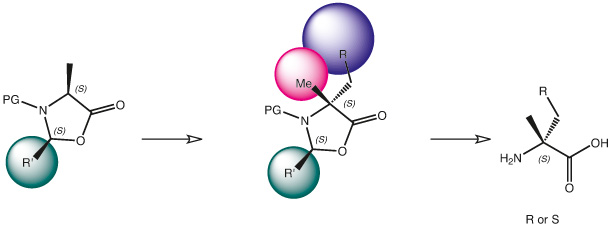
Such building blocks are being used to build up resistance towards proteolytic digestion of corresponding peptides or to implement certain amid bind conformation.
An alternative are β,β-dimethylated amino acids, where structure of the α-carbon is being maintained and the stability providing property comes through derivatization at the neighbouring carbon.
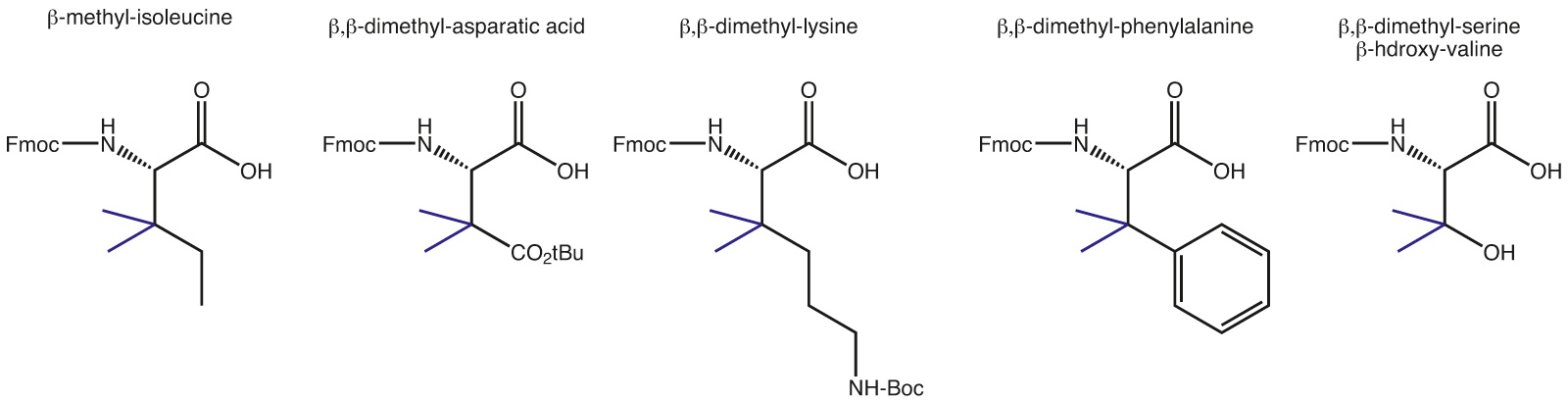
With tert-leucine and penicillamine there are already derivatives known in the peptide community, however, access to other analogues is very unique and available only through our specific synthesis platform.
References: