Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 02/05/2016
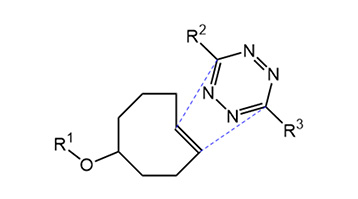
Click Chemistry is often the method of choice for bioorthogonal labeling and crosslinking. However, for biological applications, the classical Cu-promoted 1,3-dipolar cycloaddition developed by Meldal and Sharpless is often not the ideal choice due to the cytotoxicity of copper. The copper-free strain-promoted alkyne-azide cycloaddition (SPAAC, developed by Bertozzi) utilizing cyclooctynes, on the other hand, is limited by its moderate reaction kinetics for the application in biological systems, where the concentration of biomolecules is usually very low. Another limitation of cyclooctynes is the extensive patent coverage.
Tetrazine ligation offers a new copper-free, rapid and fully bioorthogonal type of click chemistry. Mechanistically, this reaction proceeds via an inverse electron-demand Diels-Alder cycloaddition reaction between a trans-cyclooctene (TCO) and a tetrazine, followed by a retro-Diels-Alder reaction under elimination of N2.
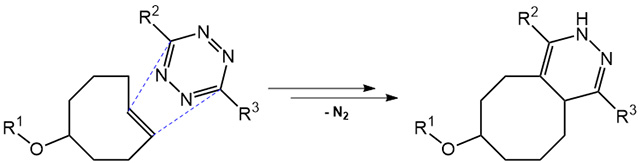
This method excels at very low concentrations (e.g. in biological systems) due to the extremely rapid second order reaction kinetics (between approx. 800 M-1s-1 and 30000 M-1s-1). Moreover, the tetrazine-TCO ligation can be performed in aqueous media and has been applied in live cell imaging. These properties make tetrazine click chemistry the method of choice for labeling or crosslinking biomolecules in living cells.
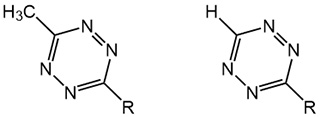
There are two main types of tetrazines that are widely applied: 6-Methyl-substituted tetrazines and 6-hydrogen-substituted tetrazines. Methyl-substituted tetrazines exhibit a high stability even when dissolved in aqueous media, while still offering faster reaction kinetics with TCO derivatives than any other bioorthogonal reaction pairs (approx. 1000 M-1 s-1). Moreover, they tolerate a wide array of reaction conditions. This makes them the prime choice for applications like protein labeling. Hydrogen-substituted tetrazines, on the other hand, show lower stability and less tolerance to harsh reaction conditions, but offer extremely fast reaction kinetics (up to 30000 M-1 s-1) for applications like in vivo imaging.
Tetrazines equipped with alkyl spacers are suitable for reactions in organic solvents. For experiments in aqueous media, however, PEG spacers are usually the best choice. Moreover, tetrazines equipped with PEG-spacers are ideal for the functionalization of proteins, since they reduce the aggregation of labeled polypeptides.
References