Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 06/03/2023

PEGylated proteins were the first polymer therapeutic drugs reaching market approval around 1990. Poly(ethylene glycol)s (PEGs) themselves show a spectrum of unique physical and chemical properties which have been described in literature extensively. The most common ones are:
➔ PEG fragments can be attached to many different positions in a protein. They can also serve as spacer or cross linker between two moieties. Amino groups of any solvent accessible lysines as well as the N-termini are the most prominent candidates for conjugation - together with thiol functions of cysteines. The C-terminus or carboxylic groups from aspartic acid and glutamic acid are also accessible for conjugation, however, are less frequently used.
➔ PEG provides high solubility in both hydrophilic and hydrophobic solvents and does not contain charged side chains. PEGs are highly mobile in water with high exclusion volume and large hydrodynamic radius.
➔ PEG is FDA-approved for internal application.
➔ PEG derivatives are available from uniform molecules with short chain lengths (down to two ethylene oxide units only), to long disperse constructs, allowing regio-specific chemical conjugation with small molecules, proteins, peptides, and biopharmaceuticals through their broad variety of available terminal chemical groups.
As PEGs render proteins tolerogenic and less immunogenic, reduce the rate of renal clearance through the kidney, increase cell permeability and alter the electroosmotic flow, they are preferred conjugation partners to improve overall pharmacokinetic properties of biopharmaceuticals.
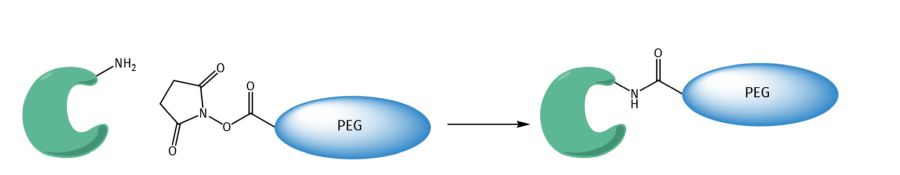
PEGylation of biopharmaceuticals can easily be achieved by reaction of the epsilon-amino functions of surface accessible lysines with active esters of PEG-acids, e.g. with N-hydroxysuccinamide, pentafluorophenol, tetrafluorophenol, or benzotriazole.
PEG-acids can usually be derived from different base carboxylic acids. Another option is to extend the PEG terminus by succinic acid. The most common PEG-acid is bearing a terminal propionic acid moiety, as the Michael addition of acrylic acid derivatives to the terminal PEG-alcohol is a high-yielding, fast reaction. However, it must be kept in mind that this moiety can undergo a retro-Michael addition and fragmentize under certain conditions. Thus, in pharmaceutical applications it is mandatory to screen for related impurities. As more stable option, acetic acid or butyric acid analogues can be used instead.
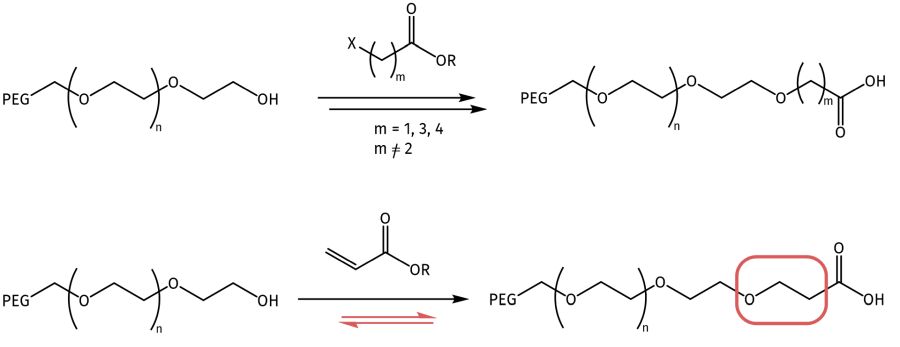
PEG-acids are usually being designed by ether bond formation with the terminal PEG-alcohol function and omega-halogen carboxylic acid derivatives or acrylic acid derivatives in the case of PEG-propionic acid. The latter one can undergo a retro-Michael addition and fragmentize.
In the following table, we have listed the relative reactivity of different NHS esters.
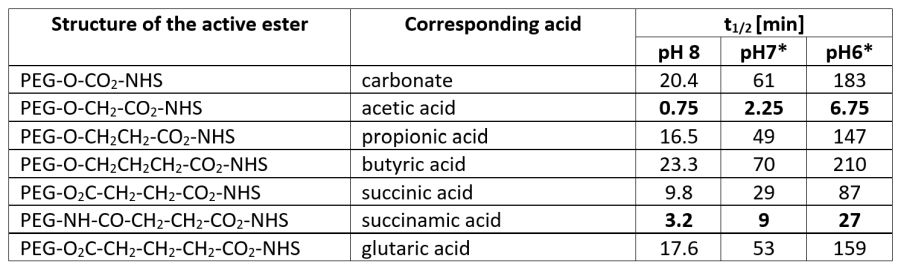
Reactivity of PEG-NHS active esters towards aminolysis at 25°C. * data for pH 7 and pH 6 were calculated based on data for pH 8. The relative reactivity of hydrolysis compared to aminolysis shows the same series, however, hydrolysis is much slower.
However, in aqueous media, high reactivity is not necessarily an advantage as simple hydrolysis of the active ester is competing with the amide bond formation. Thus, reaction yields can be optimized by wisely selecting appropriate pH, co-solvent, and carboxylic acid analogue.
➔ Check-out our webshop for available Amino-PEG-Acids!
➔ For more information about PEGylation, download our brochure!
➔ Looking for alternatives drug delivery polymers? Find suitable options in our brochure about Polymer Therapeutics!
References:
Polyethylene glycol-based linkers as hydrophilicity reservoir for antibody-drug conjugates; T. Tedeschini, B. Campara, A. Grigoletto, M. Bellini, M. Salvalaio, Y. Matsuno, A. Suzuki, H. Yoshioka, G. Pasut; Journal of Controlled Release 2021; 337: 431-447. https://doi.org/10.1016/j.jconrel.2021.07.041
The role and impact of polyethylene glycol on anaphylactic reactions to COVID-19 nano-vaccines; P. Bigini, M. Gobbi, M. Bonati, A. Clavenna, M. Zucchetti, S. Garattini, G. Pasut; Nature Nanotechnology 2021; 16: 1169-1171. https://doi.org/10.1038/s41565-021-01001-3
PEG hydration and conformation in aqueous solution: Hints to macromolecular crowding; S. Di Fonzo, B. Bellich, A. Gamini, N. Quadri, A. Cesàro; Polymer 2019; 175: 57-64. https://doi.org/10.1016/j.polymer.2019.05.004
Chapter 6 - Heterobifunctional Crosslinkers; G. T. Hermanson; Bioconjugate Techniques (Third Edition) G. T. Hermanson 2013: 299-339. https://doi.org/10.1016/B978-0-12-382239-0.00006-6
Peptide and Protein PEGylation III: Advances in Chemistry and Clinical Applications; F. M. Veronese, J. M. Harris; Advanced Drug Delivery Reviews 2008; 60: 1-88.
PEGylation - The Magic Wand. Turning Proteins and other Biopharmaceuticals into Super Performing Block Busters; T. Bruckdorfer; PharManufacturing 2007; 1: 34-41.
PEGylation, successful approach to drug delivery; F. M. Veronese, G. Pasut; Drug Discov Today 2005; 10: 1451-8. https://doi.org/10.1016/S1359-6446(05)03575-0
Chemistry for peptide and protein PEGylation; M. J. Roberts, M. D. Bentley, J. M. Harris; Adv Drug Deliv Rev 2002; 54: 459-76. https://doi.org/10.1016/S0169-409X(02)00022-4
PEGylated antibodies and antibody fragments for improved therapy: a review; A. P. Chapman; Adv Drug Deliv Rev 2002; 54: 531-45. https://doi.org/10.1016/S0169-409X(02)00026-1
Synthesis and characterization of poly(ethylene glycol) derivatives; J. M. Harris, E. C. Struck, M. G. Case, M. S. Paley, M. Yalpani, J. M. Van Alstine, D. E. Brooks; Journal of Polymer Science: Polymer Chemistry Edition 1984; 22: 341-352. https://doi.org/10.1002/pol.1984.170220207