Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 16/05/2023

Click Chemistry describes a class of 1,3 dipolar cycloadditions in which organic azides react with alkynes, catalyzed by copper (I), to form 1,2,3 triazoles. In 2022, the discovery and exploration of this reaction has been awarded with the Nobel Prize for Chemistry to Barry Sharpless, Morten P. Meldal, and Carolyn Bertozzi. In practice, click chemistry is a very versatile method to link molecules, e.g., to conjugate small molecules or payloads to proteins in a controlled fashion.
The requirement of a heavy metal ion represents a major disadvantage of the original (1st generation) copper catalyzed azide-alkyne click chemistry (CuAAC). It is hindering its application in biochemical contexts due to high toxicity towards cells as well as the detrimental oxidation of proteins. To overcome these limitations, metal-free strain promoted azide-alkyne cycloaddition (SPAAC, 2nd Generation click chemistry) and inverse electron demand diels-alder reactions (IEDDA, 3rd Generation click chemistry) have been developed.
In contrast to the original CuAAC, SPAAC is bioorthogonal, which means that it may be applied in living systems and in biochemical contexts without the risk of undesired reactions, i.e., without interfering with native biochemical processes. However, many SPAAC reagents like DBCO are rather hydrophobic, and thus require organic co-solvents. Besides, they are often reacting rather slow and thus frequently do not yield satisfactory results.
Iris Biotech is offering an advanced strained dienophile for SPAAC: Tetramethylthioheptynesulfoximine (TMTHSI), dubbed "CliCr®". CliCr® is highly reactive and dissolves well in aqueous solvents. In general, the solubility profile is mainly determined by attached moieties.
In comparison to other SPAAC reagents, CliCr® reacts extremely fast and is characterized by a very high hydrophilicity. The reaction rate of benzyl azide and CliCr® was determined to be 0.8 M-1s-1. The k value of BCN-OH was 0.14 M-1s-1. This reaction rate was observed by mass spectrometry and showed that CliCr® is one of the most reactive click reagents available nowadays. It is over 5-fold more reactive than BCN and 2.5-fold more reactive than DBCO.
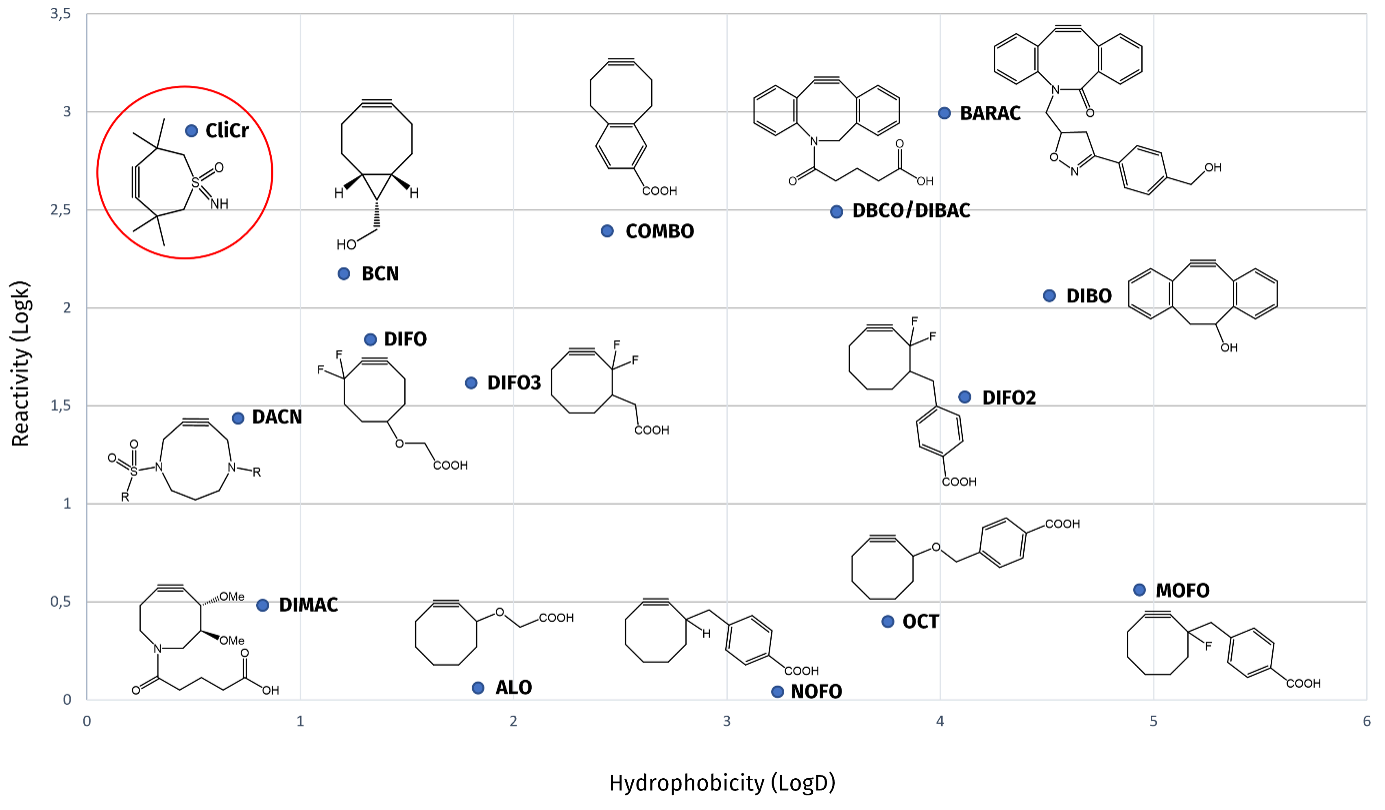
Reactivity and solubility chart of CliCr® and other SPAAC molecules. CliCr® excels through high reactivity (high log k values) and high solubility in aqueous systems (low logD values).
Upon derivatization of CliCr® with a broad range of linkers, superior SPAAC reagents are generated to be reacted with azides. Based on the fast reaction kinetics and attractive properties, the conversions and yields are high, which is also very beneficial from a cost-of-goods perspective.
Iris is offering the CliCr® base compound (RL-4180), amino-functionalized CliCr®-beta-Ala-NH2*TFA with a beta-alanine spacer (RL-4190), and CliCr®-Suc, which has been carboxy-functionalized with succinic acid (RL‑4200) as building blocks. For future IND projects, the manufacturing process to generate CliCr base compound and its derivatives already have been scaled-up and made GMP-compatible.
CliCr® has been successfully applied in the dye labeling of core-cross-linked polymeric micelles (CCPM), with peptides and proteins, and nucleic acids. Multiple applications in biochemical, aqueous environments are pursued, such as the construction of antibody drug conjugates (ADCs), peptide-conjugates, and the conjugation of chelator moieties for the incorporation of radioisotopes in theranostic applications.
→ You need more information? Join our online workshop on June 21st at 3 pm CEST to gather details about the CliCr® technology!
→ You are interested in customized CliCr® derivatives? Please inquire!
→ Check-out our Click Chemistry brochure and discover our whole click-chemistry portfolio!
CliCr® is provided under an intellectual property license from Cristal Therapeutics. The trademark CliCr® is the property of the Supplier. The transfer of this product is conditioned on the buyer using the purchased product solely in research conducted by the buyer, excluding contract research or any fee for service research, and the buyer must not (1) use this product or its components for (a) diagnostic, therapeutic or prophylactic purposes; (b) testing, analysis or screening services, or information in return for compensation on a per-test basis; or (c) manufacturing or quality assurance or quality control, and/or (2) sell or transfer this product or its components for resale, whether or not resold for use in research. For information on purchasing a license to this product for purposes other than as described above, contact Cristal Therapeutics, Oxfordlaan 55, 6229 EV Maastricht partnering@cristaltherapeutics.com.
References:
TMTHSI, a superior 7-membered ring alkyne containing reagent for strain-promoted azide-alkyne cycloaddition reactions; J. Weterings, C. J. F. Rijcken, H. Veldhuis, T. Meulemans, D. Hadavi, M. Timmers, M. Honing, H. Ippel, R. M. J. Liskamp; Chem. Sci. 2020; 11: 9011-9016. https://doi.org/10.1039/d0sc03477k
Orthogonal Covalent Entrapment of Cargo into Biodegradable Polymeric Micelles via Native Chemical Ligation; E. R. Hebels, F. Bindt, J. Walther, M. van Geijn, J. Weterings, Q. Hu, C. Colombo, R. Liskamp, C. Rijcken, W. E. Hennink, T. Vermonden; Biomacromolecules. 2022; https://doi.org/10.1021/acs.biomac.2c00865
https://cristaltherapeutics.com/technology/clicr-copper-free-click-chemistry-reagent/