Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 18/04/2023

Everyone knows the burning sensation after eating a chili. The reason therefore is 8-methyl-N-vanillyl-6-nonenamide – commonly known as Capsaicin, a chemical irritant and neurotoxin for humans and mammals in general. It interacts with the vanilloid receptor subtype 1, a non-selective cation channel expressed for example in sensory nerve fibers in the tongue. Upon activation of the receptor, cations can pass through the cell membrane, resulting in depolarization of the neuron and signaling to the brain. In addition to Capsaicin, this receptor is also activated by noxious thermal heat. Thus, the spiciness of hot peppers is perceived as burning sensation.
As pharmaceutical agent Capsaicin is used in topical ointments and dermal patches to treat various forms of pain and circulatory disorders.
From a conjugation point of view, Capsaicin bears a single phenol function allowing linker attachment via an initial carbamate. In this example, a double self-immolative sequence leads to fragmentation and release of oxathiolone and dimethylimidazolidinone.

Linker-conjugated Capsaicin undergoes self-immolative fragmentation upon reduction.
Huperzine A is a naturally occurring alkaloid mainly found in the chinese firmoss Huperzia serrata. It acts as highly active, reversible acetylcholinesterase inhibitor, thus preventing the decomposition of the neurotransmitter acetylcholine. Besides, it is reported to improve cognitive function. In China, already in 1994, Huperzine A was approved for the treatment of Alzheimer’s disease and is supposed to improve cognitive function. Conjugating Huperzine A to suitable vectors such as antibodies or nanoparticles might allow to improve its pharmacological effects.
Huperzine A carries a single amino function, which can be directly conjugated to benzyl carbamate decorated with a suitable fragmentation trigger such as Val-Cit or Val-Ala.
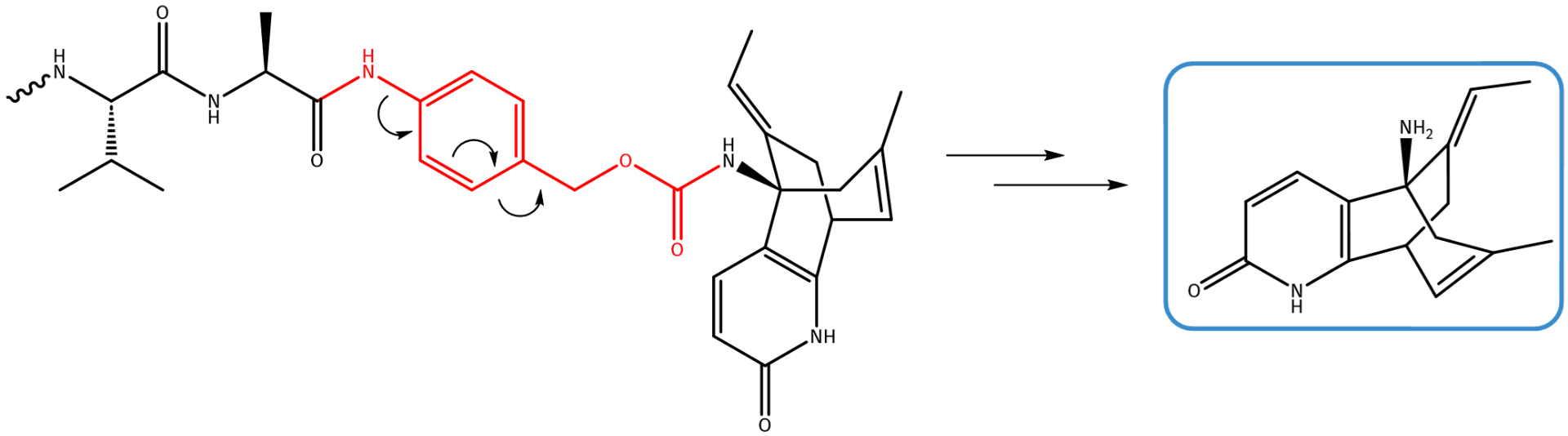
Linker-conjugated Huperzine A undergoes self-immolative fragmentation in the presence of Cathepsin B.
Lithocholic acid (LCA) is a bile acid generated from chenodeoxycholid acid by bacteria in the gut. While originally known as detergent to facilitate the digestion and absorption of lipids, LCA is meanwhile reported as weak Vitamin D receptor ligand, as potential anti-aging compound, and in the context of apoptosis during early stages of carcinogenesis.
Lithocholic acid carries a single carboxylic acid function, which makes it ideal for ester formation with p-hydroxybenzyl alcohol, which – for example – can be decorated with a pH sensitive self-immolative silyl ether function.
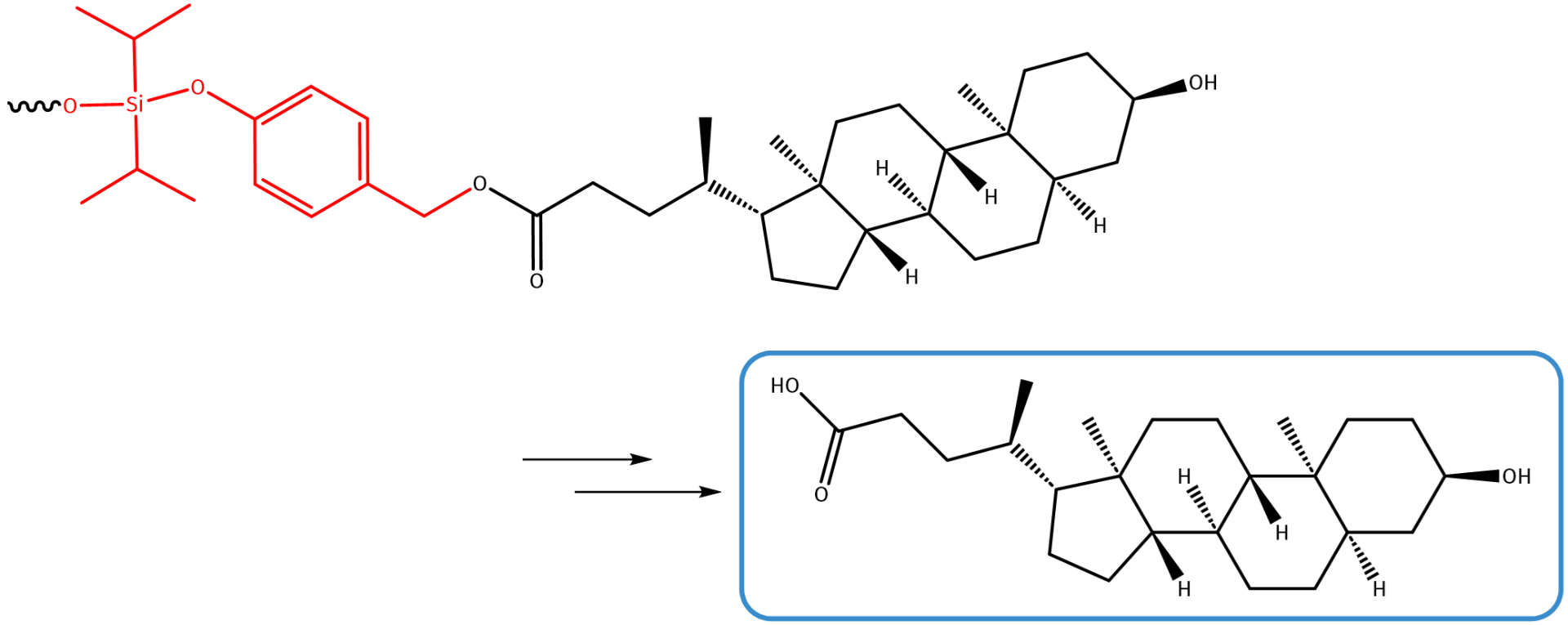
Linker-conjugated lithocholic acid undergoes self-immolative fragmentation under acidic conditions.
→ For more details on the conjugation of natural products, click here!
→ Interested in linker technologies? Download our brochure Linkerology®
References:
The effect of lithocholic acid on proliferation and apoptosis during the early stages of colon carcinogenesis: differential effect on apoptosis in the presence of a colon carcinogen; V. Kozoni, G. Tsioulias, S. Shiff, B. Rigas; Carcinogenesis 2000; 21: 999-1005. https://doi.org/10.1093/carcin/21.5.999
Old Chinese herbal medicine used for fever yields possible new Alzheimer disease therapy; A. A. Skolnick; JAMA 1997; 277: 776. https://doi.org/10.1001/jama.1997.03540340010004
[+]-Huperzine A treatment protects against N-methyl-D-aspartate-induced seizure/status epilepticus in rats; B. R. Coleman, R. H. Ratcliffe, S. A. Oguntayo, X. Shi, B. P. Doctor, R. K. Gordon, M. P. Nambiar; Chem Biol Interact 2008; 175: 387-95. https://doi.org/10.1016/j.cbi.2008.05.023
Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses; V. Fattori, M. S. Hohmann, A. C. Rossaneis, F. A. Pinho-Ribeiro and W. A. Verri; Molecules 2016; 21: 844. https://doi.org/10.3390/molecules21070844
Efficacy and safety of natural acetylcholinesterase inhibitor huperzine A in the treatment of Alzheimer’s disease: an updated meta-analysis; B. Wang, H. Wang, Z. Wei, Y. Song, L. Zhang, H. Chen; J. Neural Transm 2009; 116: 457-465. https://doi.org/10.1007/s00702-009-0189-x
Lithocholic Acid Is a Vitamin D Receptor Ligand That Acts Preferentially in the Ileum; M. Ishizawa, D. Akagi, M. Makishima; Int. J. Mol. Sci. 2018; 19(7): 1975. https://doi.org/10.3390/ijms19071975
Lithocholic Acid Derivatives as Potent Vitamin D Receptor Agonists; H. Sasaki, H. Masuno, H. Kawasaki, A. Yoshihara, N. Numoto, N. Ito, H. Ishida, K. Yamamoto, N. Hirata, Y. Kanda, E. Kawachi, H. Kagechika, A. Tanatani; J. Med. Chem. 2021; 64(1): 516-526. https://doi.org/10.1021/acs.jmedchem.0c01420
Lithocholic bile acid induces apoptosis in human nephroblastoma cells: a non-selective treatment option; J. Trah, J. Arand, J. Oh, L. Pagerols-Raluy, M. Trochimiuk, B. Appl, H. Heidelbach, D. Vincent, M. A. Saleem, K. Reinshagen, A. K. Mühlig, M. Boettcher; Scientific Reports 2020; 20: 20349. https://doi.org/10.1038/s41598-020-77436-w
Research Progresso f Bile Acids in Cancer; J. Fu, M. Yu, W. Xu, S. Yu; Front. Oncol. 2022; 11: 2021. https://doi.org/10.3389/fonc.2021.778258