Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 30/05/2023

In our first blog about Conjugating Natural Products, we already highlighted the importance of natural products in drug discovery. This fact is not limited to terrestrial plants and microorganisms but also counts for marine natural products (MNPs) and marine organisms.
The first living organisms appeared in the sea more than 3,500 million years ago. Oceans cover around two thirds of the earth’s surface and bear rich biodiversity. The harsh conditions prevailing in the deep sea (e.g. darkness, pressure, salinity) lead to the development of unique adaption and defense strategies within all marine species and also to the creation of a great variety of chemical molecules with distinct properties.
Cone snails have gained attention in the recent years, however, numerous more compounds have been identified in fish, tunicate, sponge, mollusk, worm, fungus, bacterium, soft coral, and bryozoa. Thus, it is not surprising that by end of 2016 almost 30,000 MNPs had been identified and the potential behind is inestimable. Application fields range from cancer therapy over chronic pain to food supplements. As reported beginning of 2022 (https://www.marinepharmacology.org), 20 drugs from marine sources are in clinical use, e.g. for the treatment of various types of cancer, Alzheimer’s disease, wound healing, cognition, schizophrenia, and hypertriglyceridima. Amongst them, six APIs are already approved by the FDA, two compounds are in Phase III, five are in Phase II and seven in Phase I. Major success has been achieved in the development of cancer- and ADC-related drugs.
Shown below is a marine penicillium from a chordate (Bergen, Norway) which has anti-inflammatory properties and a logP of 2.04, hence, it nicely fulfills the Lipinski rule of five – a rule of thumb to evaluate “druglikeness” which says that the logP value of a potential drug should not exceed five. The free carboxylic acid function can be addressed by formation of a benzyl ester, which can be substituted by a bisisopropyl silyl ether. Such silyl ethers are stable during circulation in plasma, however, undergo fragmentation, if the pH is lowered (e.g. to 6.5 as present in the early endosomes or pH 4.5 as present in lysosomes) thus releasing the drug compound in a traceless manner.
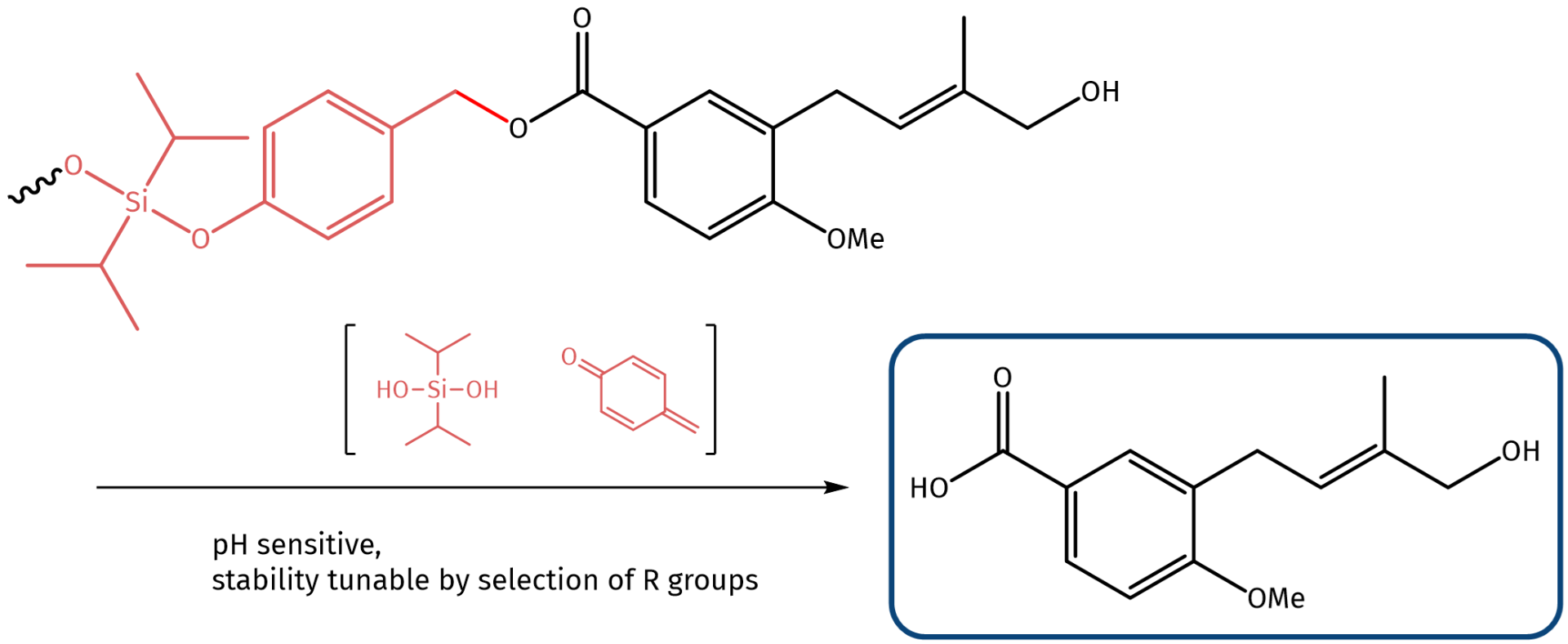
Marine penicillium from chordate decorated with a silyl ether which undergoes self-immolative fragmentation at low pH.
Another example is a compound found in a marine fungus from a seaweed (Esukadi, Spain). It has a logP value of 2.45. Via its hydroxy group, this MNP can be decorated with a double self-immolative linker fragmentizing into 1,3-oxathiolan-2-one and 1,3-dimethylimidazolidin-2-one once the cleavage is triggered by reduction of the disulfide moiety to the free thiols.
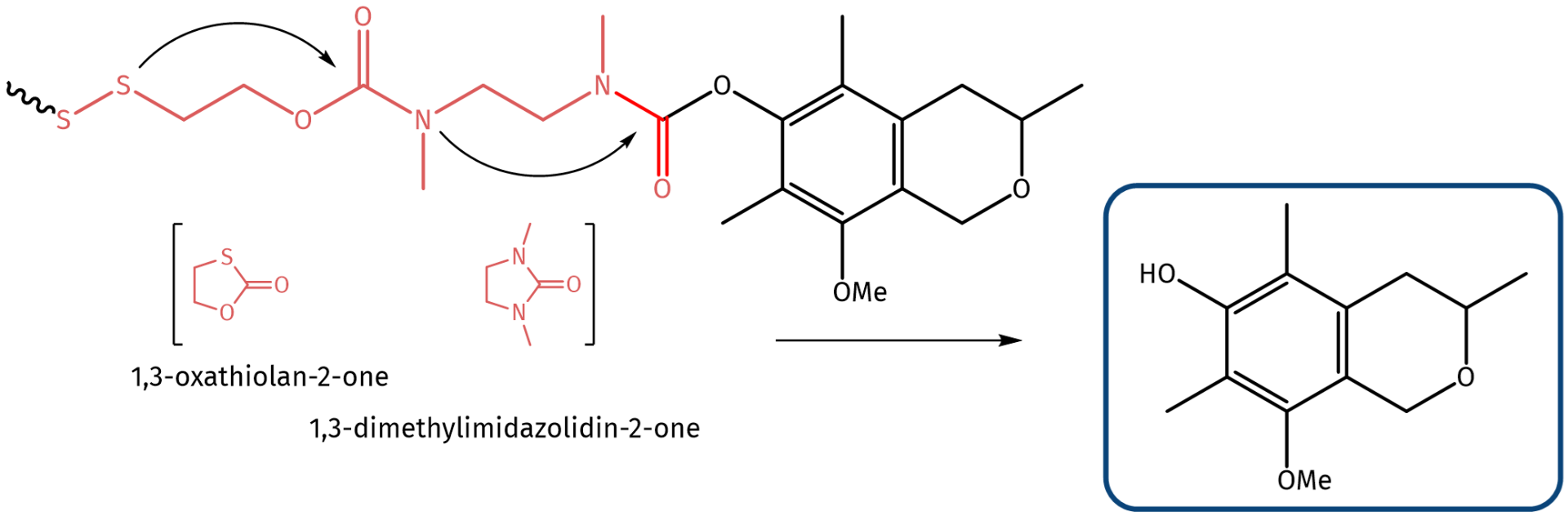
MNP decorated with a double self-immolative linker, which fragmentizes upon disulfide reduction.
→ For more details on the conjugation of marine natural products, click here!
→ Interested in linker technologies? Download our brochure Linkerology®
References:
New Marine Derived Anticancer Therapeutics ─ A Journey from the Sea to Clinical Trials; J. Jimeno, G. Faircloth, J. F. Sousa-Faro, P. Scheuer, K. Rinehart; Marine Drugs 2004; 2: 14-29. https://doi.org/10.3390/md201014
https://www.marinepharmacology.org
Marine Natural Products in Clinical Use; N. Haque, S. Parveen, T. Tang, J. Wei, Z. Huang; Mar Drugs 2022; 18(20): 528. https://doi.org/10.3390/md20080528
Marine Natural Products in Medicinal Chemistry; C. Jiménez; ACS Med. Chem. Lett. 2018; 9(10): 959-961. https://doi.org/10.1021/acsmedchemlett.8b00368
Marine natural products; A. R. Carroll, B. R. Copp, R. A. Davis, R. A. Keyzers, M. R. Prinsep; Nat. Prod. Rep. 2023; 40: 275-235. https://doi.org/10.1039/D2NP00083K
Marine natural products; A. R. Carroll, B. R. Copp, R. A. Davis, R. A. Keyzers, M. R. Prinsep; Nat. Prod. Rep. 2021; 38: 362-413. https://doi.org/10.1039/D0NP00089B