Interested in histidine derivatives? Iris Biotech provides a selection of deamino- and decarboxy-products. Find out more about benefits and potential applications of our new building blocks.
The essential amino acid Histidine (His, H), first isolated in 1896 by Albrecht Kossel and Sven Gustaf Hedin, bears an imidazole side chain which plays a crucial role for the activity of many enzymes. It can act as a proton shuttle and with its two N-donors it is one of the most effective ligation sites in metallopeptides.
An example for an essential histidine-bearing peptide is the glucagon-like peptide-1 (GLP-1), which controls the secretion of insulin in a glucose-dependent manner. Truncation studies on its sequence revealed the importance of the histidine residue at position 7 for receptor recognition, function, and the peptide’s insulinotropic activity. As native GLP-1 is rapidly degraded, the modified analogues Liraglutide and Semaglutide – also bearing this histidine residue – represent essential treatments for type 2 diabetes.
Sequence of the glucagon-like peptide-1 (7-37) amide:
H-His-Ala-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly-Gln-Ala-Ala-Lys-Glu-Phe-Ile-Ala-Trp-Leu-Val-Lys-Gly-Arg-Gly-NH2
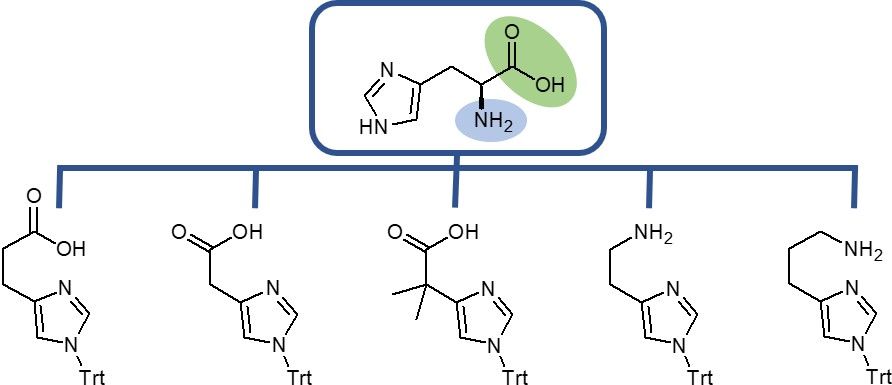
Iris Biotech offers a selection of deamino- and decarboxy-histidine derivatives, which can be used as tools for the preparation of libraries to investigate structure activity relationships leading to the generation of drugs with improved pharmacokinetics. As especially histidines are prone to racemization during peptide synthesis, the installation of deamino- or decarboxy-His at the C- or N-terminus of a peptide brings another crucial advantage as it allows to circumvent this issue due to the lack of chirality. Thus, this measure reduces the amount of undesired side-products, which might be difficult to remove.
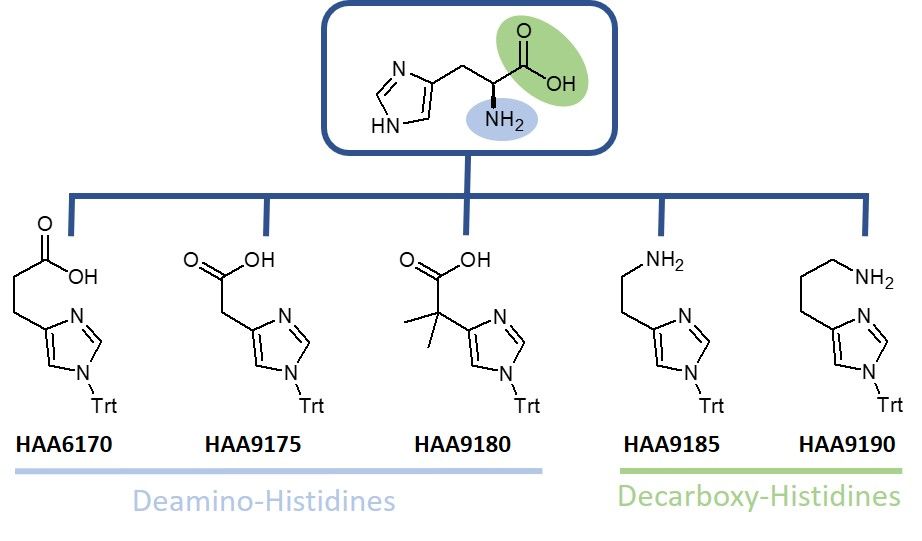
For deamino-histidines, Iris Biotech provides the trityl protected imidazole propionic acid HAA6170, its shorter chain imidazole-4-acetic acid HAA9175 and the demethylated derivative thereof (HAA9180). HAA9175 is reported as building block for the synthesis of imidazolyl-polyethyleneimine modified nanoparticles benefiting from increased transfection efficiency as well as for the synthesis of ribonuclease mimics. Furthermore, imidazole-4-acetic acid is suggested as antagonist of GABAC receptors and partial GABAAR agonist being capable of penetrating the blood-brain barrier in vivo.
Decarboxy-histidine HAA9185 represents a trityl protected histamine and is reported as component of potent agonists of the histamine H2 and H4 receptor. It can be placed at the C-terminus of a peptide replacing natural histidine and is reported within the synthesis of truncated GLP-1 based peptides to analyze metabolic stability and pharmacokinetic properties. Other reports highlight HAA9185 as building block for potent inhibitors of farnesyltransferase and geranylgeranyltransferase-I. Its longer chain derivative HAA9190 is highlighted as fragment of selective H2R and H4R agonists and for its inhibitory activity towards the H3 receptor and the histamine N-methyltransferase.
References:
- Histidine: A Systematic Review on Metabolism and Physiological Effects in Human and Different Animal Species; J. Moro, D. Tomé, P. Schmidely, T.-C. Demersay, and D. Azzout-Marniche; Nutrients 2020; 12: 1414. https://doi.org/10.3390/nu12051414. +
- Structural and Kinetic Characterization of Active-Site Histidine as a Proton Shuttle in Catalysis by Human Carbonic Anhydrase II; Z. Fisher, J. A. Hernandez Prada, C. Tu, D. Duda, C. Yoshioka, H. An, L. Govindasamy, D. N. Silverman, R. McKenna; Biochemistry 2005; 44: 1097-1105. https://doi.org/10.1021/bi0480279.
- Stability of some Metal Complexes of Histidine; L. E. Maley, D. P. Mellor; Nature 1950; 165: 453. https://doi.org/10.1038/165453a0.
- Comparison of the effects of various C-terminal and N-terminal fragment peptides of glucagon-like peptide-1 on insulin and glucagon release from the isolated perfused rat pancreas; S. Suzuki, K. Kawai, S. Ohashi, H. Mukai, K. Yamashita; Endocrinology 1989; 125: 3109-14. https://doi.org/10.1210/endo-125-6-3109.
- In Vivo Protein Binding of Liraglutide in Human Plasma Determined by Reiterated Stepwise Equilibrium Dialysis; A. Plum, L. B. Jensen, J. B. Kristensen; Journal of Pharmaceutical Sciences 2013; 102: 2882-2888. https://doi.org/10.1002/jps.
- Glucagon-like Peptide-1 (GLP-1) Analogs: Recent Advances, New Possibilities, and Therapeutic Implications; B. Manandhar, J.-M. Ahn; J. Med. Chem. 2015; 58: 1020-1037. https://doi.org/10.1021/jm500810s.
- 1,4-Butanediol diglycidyl ether (BDE)-crosslinked PEI-g-imidazole nanoparticles as nucleic acid-carriers in vitro and in vivo; R. Goyal, R. Bansal, S. Tyagi, Y. Shukla, P. Kumar and K. C. Gupta; Mol Biosyst 2011; 7: 2055-65. https://doi.org/10.1039/c1mb05049d
- Synthesis and cleavage experiments of oligonucleotide conjugates with a diimidazole-derived catalytic center; B. Verbeure, C. J. Lacey, M. Froeyen, J. Rozenski and P. Herdewijn; Bioconjug Chem 2002; 13: 333-50. https://doi.org/10.1021/bc0155622
- Pharmacology and Function of Imidazole 4-Acetic Acid in Brain; General Pharmacology: The Vascular System; 1998: 31: 503-509. https://doi.org/10.1016/S0306-3623(98)00079-2.
- Discovery of alpha-Substituted Imidazole-4-acetic Acid Analogues as a Novel Class of rho1 gamma-Aminobutyric Acid Type A Receptor Antagonists with Effect on Retinal Vascular Tone; J. Krall, B. M. Brygger, S. B. Sigurethardottir, C. K. Ng, C. Bundgaard, J. Kehler, B. Nielsen, T. Bek, A. A. Jensen and B. Frolund; ChemMedChem 2016; 11: 2299-2310. https://doi.org/10.1002/cmdc.201600356.
- Crystal structure of the GLP-1 receptor bound to a peptide agonist; A. Jazayeri, M. Rappas, A. J. H. Brown, J. Kean, J. C. Errey, N. J. Robertson, C. Fiez-Vandal, S. P. Andrews, M. Congreve, A. Bortolato, J. S. Mason, A. H. Baig, I. Teobald, A. S. Dore, M. Weir, R. M. Cooke and F. H. Marshall; Nature 2017; 546: 254-258. https://doi.org/10.1038/nature22800
- Acylguanidines as bioisosteres of guanidines: NG-acylated imidazolylpropylguanidines, a new class of histamine H2 receptor agonists; P. Ghorai, A. Kraus, M. Keller, C. Gotte, P. Igel, E. Schneider, D. Schnell, G. Bernhardt, S. Dove, M. Zabel, S. Elz, R. Seifert and A. Buschauer; Journal of medicinal chemistry 2008; 51: 7193-204. https://doi.org/10.1021/jm800841w
- Potent inhibitors of farnesyltransferase and geranylgeranyltransferase-I; D. N. Nguyen, C. A. Stump, E. S. Walsh, C. Fernandes, J. P. Davide, M. Ellis-Hutchings, R. G. Robinson, T. M. Williams, R. B. Lobell, H. E. Huber and C. A. Buser; Bioorg Med Chem Lett 2002; 12: 1269-73. https://doi.org/10.1016/s0960-894x(02)00154-3
- Dimeric carbamoylguanidine-type histamine H2 receptor ligands: A new class of potent and selective agonists; N. Kagermeier, K. Werner, M. Keller, P. Baumeister, G. Bernhardt, R. Seifert and A. Buschauer; Bioorg Med Chem 2015; 23: 3957-69. https://doi.org/10.1016/j.bmc.2015.01.012
- Synthesis and structure-activity relationships of cyanoguanidine-type and structurally related histamine H4 receptor agonists; P. Igel, R. Geyer, A. Strasser, S. Dove, R. Seifert and A. Buschauer; Journal of medicinal chemistry 2009; 52: 6297-313. https://doi.org/10.1021/jm900526h
- Imidazole derivatives as a novel class of hybrid compounds with inhibitory histamine N-methyltransferase potencies and histamine hH3 receptor affinities; S. Graßmann, J. Apelt, W. Sippl, X. Ligneau, H. H. Pertz, Y. H. Zhao, J.-M. Arrang, C. R. Ganellin, J.-C. Schwartz, W. Schunack and H. Stark; Bioorganic & Medicinal Chemistry 2003; 11: 2163-2174. https://doi.org/10.1016/s0968-0896(03)00120-2
- [3-(1H-imidazol-4-yl)propyl]guanidines containing furoxan moieties: a new class of H3-antagonists endowed with NO-donor properties; M. Bertinaria, A. D. Stilo, P. Tosco, G. Sorba, E. Poli, C. Pozzoli, G. Coruzzi, R. Fruttero and A. Gasco; Bioorg Med Chem 2003; 11: 1197-205. https://doi.org/10.1016/s0968-0896(02)00651-x
- Discovery of a Novel Non-Peptide Somatostatin Agonist with SST4Selectivity; M. Ankersen, M. Crider, S. Liu, B. Ho, H. S. Andersen and C. Stidsen; Journal of the American Chemical Society 1998; 120: 1368-1373. https://doi.org/10.1021/ja973325x
- Novel H3 receptor antagonists. Sulfonamide homologs of histamine; R. Wolin, M. Connolly, A. Afonso, J. A. Hey, H. She, M. A. Rivelli, S. M. Willams and R. E. West, Jr.; Bioorg Med Chem Lett 1998; 8: 2157-62. https://doi.org/10.1016/s0960-894x(98)00379-5