Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 18/10/2022

Copper – a nobel metal? Yes, of course! The 2022 Chemistry Nobel prize is awarded to B. Sharpless, M. Meldal and C. Bertozzi for their achievements in the field of Click Chemistry.
Around the turn of the century, Barry Sharpless was investigating a type of chemical transformation in which building blocks “simply” snap together. Independently of each other, he and Morten Meldal discovered the copper catalyzed azide-alkyne 1,3-dipolar cycloaddition, quickly dubbed “Click Chemistry”. The stable reaction product, a triazole, represents a highly useful structural motive which can be found in pharmaceuticals, dyes, and agricultural products, among other things. Due to its high thermodynamic driving force, which is usually greater than 20 kcal/mol, the Click reaction rapidly proceeds to completion in almost all cases.
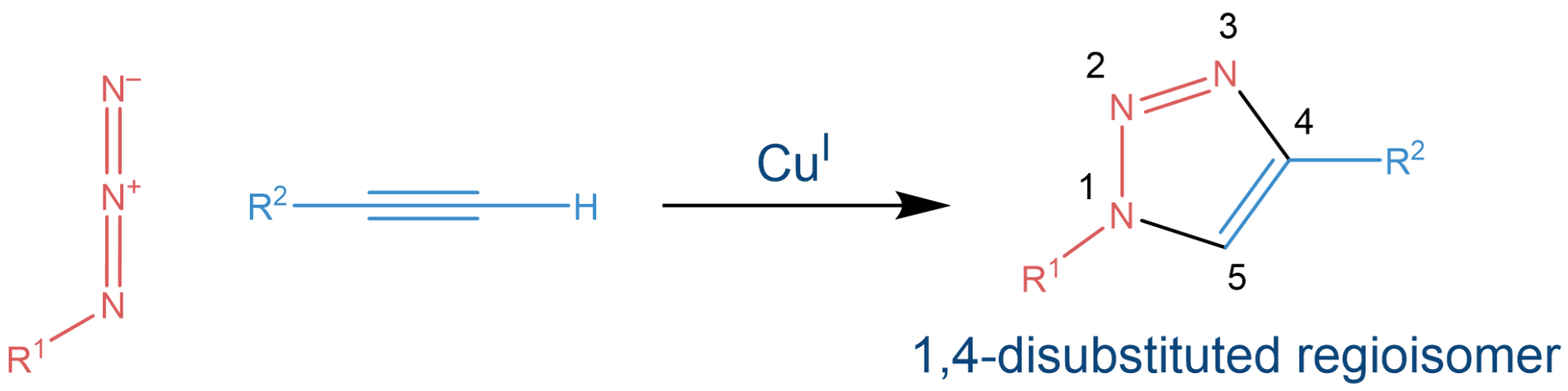
The copper-catalyzed azide-alkyne cycloaddition affords a 1,4-disubstituted triazole.
The presence of copper, however, limits in vivo applications of this reaction for several reasons such as high cell toxicity, undesired oxidation of proteins, inhibition of luminescence properties of nanocrystals.
The Click technology was then pushed to a new dimension by Carolyn Bertozzi, who developed Click reactions compatible with living organisms without interfering normal cell functioning (= bioorthogonal Click reactions). The so-called copper-free strain-promoted azide-alkyne cycloaddition (SPAAC) is based on the strong reaction of strained alkynes, e.g. DBCO, DACN, with azides. By modifying the cyclooctyne core structure with heteroatoms, fluorine substituents and fused rings, key properties such as cycloaddition kinetics, stability, solubility, and pharmacokinetics can be further optimized.
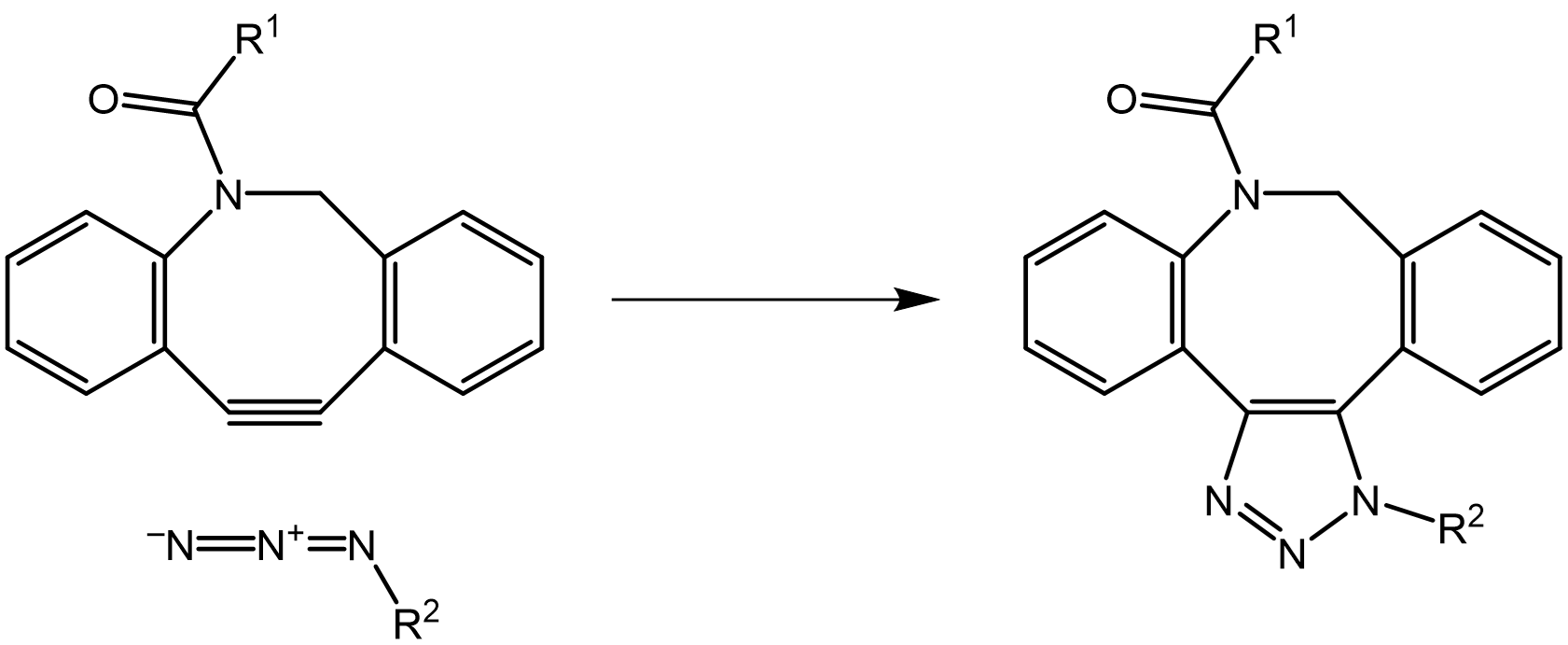
Reaction of DBCO as an exemplary strained cyclooctyne with an azide.
However, SPAAC is limited by its moderate reaction kinetics for the application in live cells, where the concentration of biomolecules is usually low. Another potential drawback of cyclooctynes is the extensive patent coverage of many variants.
Tetrazine ligation presents the option for a copper-free, rapid, and fully bioorthogonal type of Click chemistry. Mechanistically, this reaction proceeds via an inverse electron-demand Diels-Alder cycloaddition reaction between a tetrazine and a strained alkene such as trans-cyclooctene (TCO), cyclopropane or norbornene, followed by a retro-Diels-Alder reaction under elimination of dinitrogen, the latter rendering the reaction irreversible.
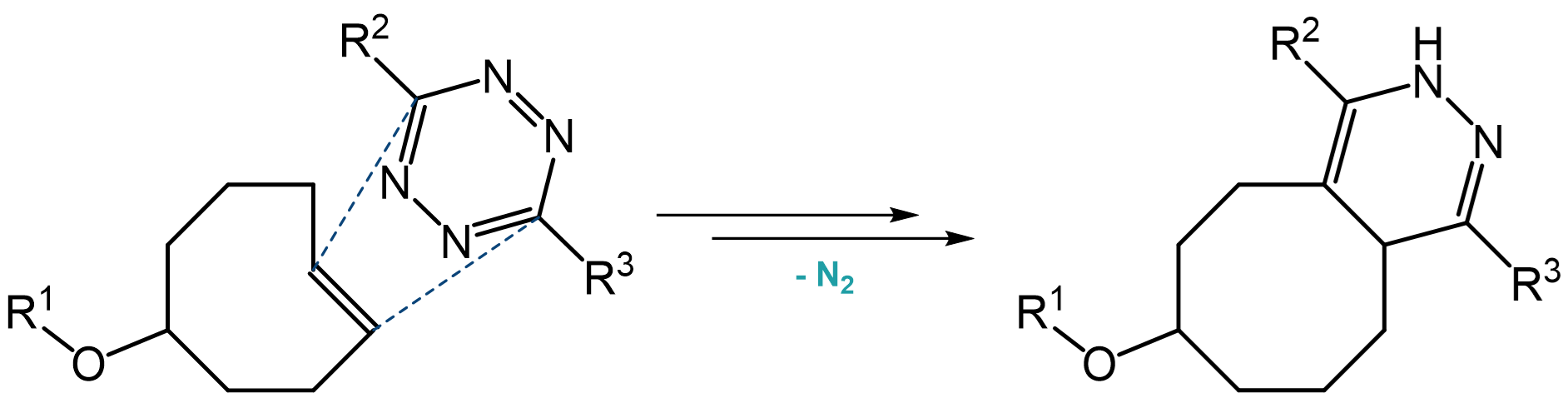
Reaction between a trans-cyclooctene (TCO) and a tetrazine.
Nowadays, applications for the Click conjugation technique range from imaging and drug design to the development of sensors, thereby covering such diverse fields as chemical biology, material science, surface and polymer chemistry.
A variety of azido and alkyne building blocks with different protecting groups as well as related required reagents, e.g. TBTA (RL-2010), THPTA (RL-2210), are available from Iris Biotech. Some of those compounds can be incorporated into peptides and proteins by recombinant syntheses, particularly by non-neutral protein translation using the amber-suppression-based orthogonal system, while others are suitable for solid phase peptide synthesis.
➔ For more detailed information and related products, please download our brochure on Click Chemistry!
➔ Interested in Click-chemistry for cell-free protein synthesis? Watch the recording of our workshop!
References:
A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes; V. V. Rostovtsev, L. G. Green, V. V. Fokin, K. B. Sharpless; Angew. Chem. Int. Ed. 2002; 41: 2596-2599. https://doi.org/10.1002/1521-3773(20020715)41:143.0.Co;2-4
CuI-Catalyzed Alkyne-Azide “Click” Cycloadditions from a Mechanistic and Synthetic Perspective; V. D. Bock, H. Hiemstra, J. H. van Maarseveen; Eur. J. Org. Chem. 2006; 2006: 51-68. https://doi.org/10.1002/ejoc.200500483
A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems; N. J. Agard, J. A. Prescher, C. R. Bertozzi; J Am Chem Soc 2004; 126: 15046-7. https://doi.org/10.1021/ja044996f
Bioconjugation with strained alkenes and alkynes; M. F. Debets, S. S. van Berkel, J. Dommerholt, A. T. Dirks, F. P. Rutjes, F. L. van Delft; Acc Chem Res 2011; 44: 805-15. https://doi.org/10.1021/ar200059z
Strain-Promoted 1,3-Dipolar Cycloaddition of Cycloalkynes and Organic Azides; J. Dommerholt, F. Rutjes, F. L. van Delft; Top Curr Chem (Cham) 2016; 374: 16. https://doi.org/10.1007/s41061-016-0016-4
Heteroatom-embedded Medium-Sized Cycloalkynes: Concise Synthesis, Structural Analysis, and Reactions; R. Ni, N. Mitsuda, T. Kashiwagi, K. Igawa, K. Tomooka; Angew. Chem. Int. Ed. 2015; 54: 1190-1194. https://doi.org/10.1002/anie.201409910
Biomedical applications of tetrazine cycloadditions; N. K. Devaraj, R. Weissleder; Acc Chem Res 2011; 44: 816-27. https://doi.org/10.1021/ar200037t
Inverse electron demand Diels-Alder (iEDDA)-initiated conjugation: a (high) potential click chemistry scheme; A. C. Knall, C. Slugovc; Chem Soc Rev 2013; 42: 5131-42. https://doi.org/10.1039/c3cs60049a
Highly accelerated inverse electron-demand cycloaddition of electron-deficient azides with aliphatic cyclooctynes; J. Dommerholt, O. van Rooijen, A. Borrmann, C. F. Guerra, F. M. Bickelhaupt, F. L. van Delft; Nat Commun 2014; 5: 5378. https://doi.org/10.1038/ncomms6378
Inverse electron demand Diels-Alder (IEDDA) reactions in peptide chemistry; M. Pagel; J. Pept. Sci. 2019; 25: e3141. https://doi.org/10.1002/psc.3141
Tetrazine-Based Cycloadditions: Application to Pretargeted Live Cell Imaging; N. K. Devaraj, R. Weissleder, S. A. Hilderbrand; Bioconjug. Chem. 2008; 19: 2297-2299. https://doi.org/10.1021/bc8004446
https://www.nobelprize.org/prizes/chemistry/2022/popular-information/?amp;amp [17.10.2022]