Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 02/05/2023

Due to their natural origin, peptide drugs often suffer from degradation by proteases, limiting their effectiveness as therapeutics. In this regard, lipidated amino acid building blocks have gained increasing interest for peptide engineering as their fatty acid alkyl chain promotes non-covalent binding to serum albumin, thereby improving the pharmacokinetic and pharmacologic properties of peptide APIs. With this strategy, long half-lives for otherwise unstable peptide APIs can be achieved, and the frequency and dosage of drug administration can typically be reduced.
The most prominent examples in this context are the marketed glucagon-like peptide-1 analog blockbuster drugs Liraglutide and Semaglutide.
In this regard, at Iris Biotech, we offer functionalized fatty di-acid derivatives as well as protected fatty di-acid modified amino acids. Thereby, the impact on half-life extension of the peptidic drug is depending on the type of modification, e.g., position of the attached fatty acid and the length of its alkyl chain. In a direct comparison, fatty di-acids typically outperform their monoacid counterparts.
Our latest portfolio additions are Fmoc-L-Lys(tBuO-Thap)-OH (FAA8990) and Fmoc-L-Lys(tBuO-Thap-L-Glu-OtBu)-OH (FAA8980).
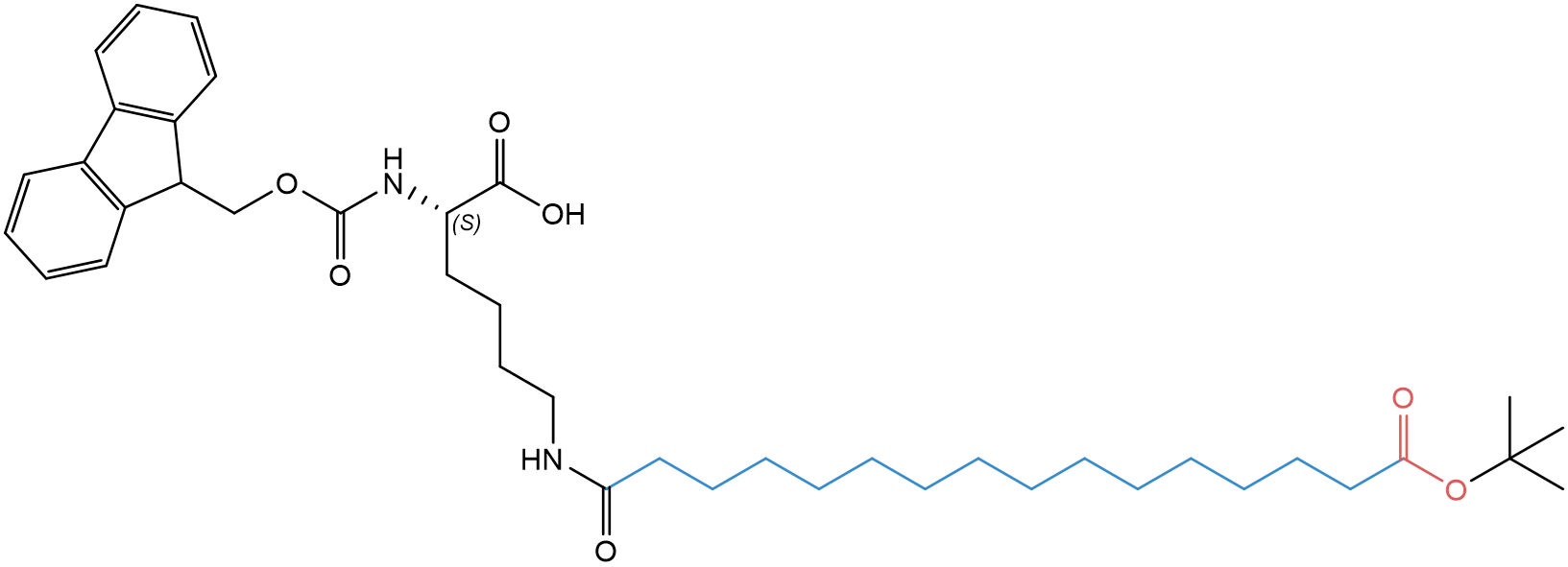
Fig. 1: Structure of Fmoc-L-Lys(tBuO-Thap)-OH (FAA8990).
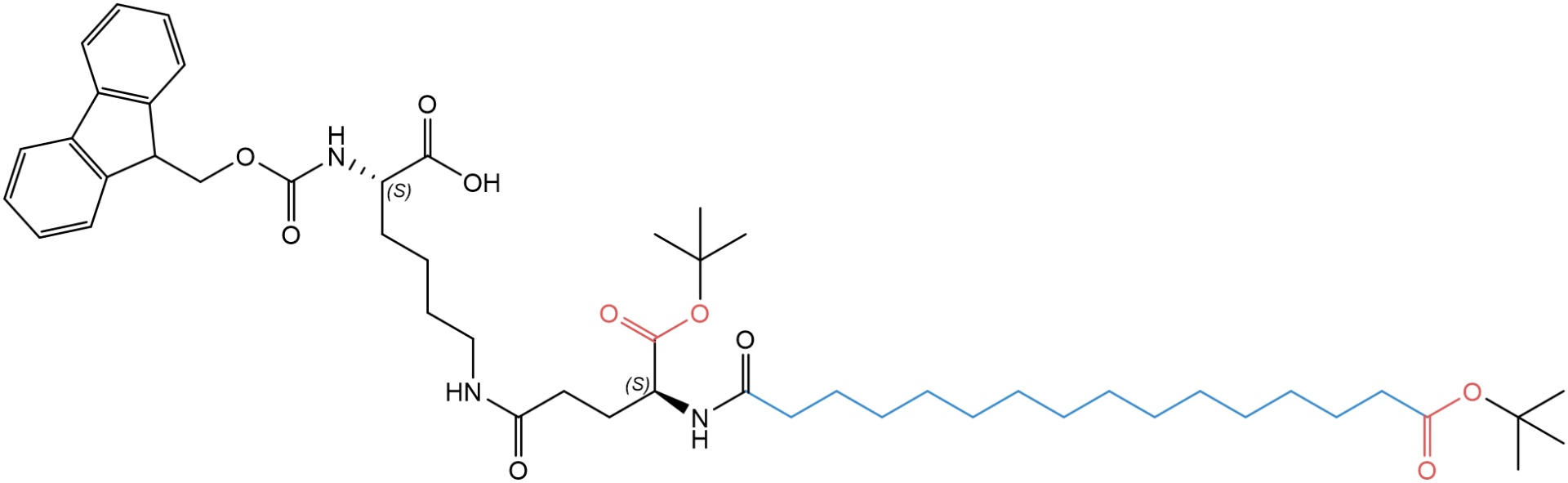
Fig. 2: Structure of Fmoc-L-Lys(tBuO-Thap-L-Glu-OtBu)-OH (FAA8980).
While FAA8990 has a C16:0 di-acid side chain with a terminal carboxyl group (hexadecanedioic acid/thapsic acid), FAA8980 has a second carboxyl group derived from an additional glutamic acid spacer. For synthetic convenience, the side chain carboxyl groups are orthogonally protected with OtBu, which can be removed as soon as the synthesis is completed.
See all our fatty acid building blocks available in our webshop or download our flyer peptide modifiers
You are interested in further technologies to increase your peptide’s stability during circulation? Discover our b,b-dimethylated amino acids, hydrolysis stable analogs, and polymers for drug delivery
References:
Synthesis of Lipidated Peptides; F. Rosi, G. Triola; Peptide Synthesis and Applications 2013; 161-189. https://doi.org/10.1007/978-1-62703-544-6_12
Synthetic lipidation of peptides and amino acids: monolayer structure and properties; P. Berndt, G. B. Fields, M. Tirrell; J. Am. Chem. Soc. 1995; 117: 9515-9522. https://doi.org/10.1021/ja00142a019
Peptide Lipidation – A Synthetic Strategy to Afford Peptide Based Therapeutics; R. Kowalczyk, P. W. R. Harris, G. M. Williams, S.-H. Yang, M. A. Brimble; Peptides and Peptide-based Biomaterials and their Biomedical Applications 2017; 1030: 185-227. https://doi.org/10.1007/978-3-319-66095-0_9
Solid-Phase Synthesis of Lipidated Peptides; G. Kragol, M. Lumbierres, J. M. Palomo; H. Waldmann; Angew. Chem. Int. Ed. 2004; 116: 5963-5966. https://doi.org/10.1002/ange.200461150
Derivatization with fatty acids in peptide and protein drug discovery; P. Kurtzhals, S. Østergaard, E. Nishimura, T. Kjeldsen; Nat. Rev. 2023; 22: 59-80. https://doi.org/10.1038/s41573-022-00529-w
The effect of fatty diacid acylation of human PYY3-36 on Y2 receptor potency and half-life in minipigs S. Østergaard, J. F. Paulsson, J. Kofoed, F. Zosel, J. Olsen, C. Bekker Jeppesen, J. Spetzler, L. Ynddal, L. Gram Schleiss, B. Østergaard Christoffersen, K. Raun, U. Sensfuss, F. S. Nielsen, R. Jørgensen, B. S. Wulff; Scientific Reports 2021; 11: 21179. https://doi.org/10.1038/s41598-021-00654-3