Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 09/02/2017
1. The Click Reaction
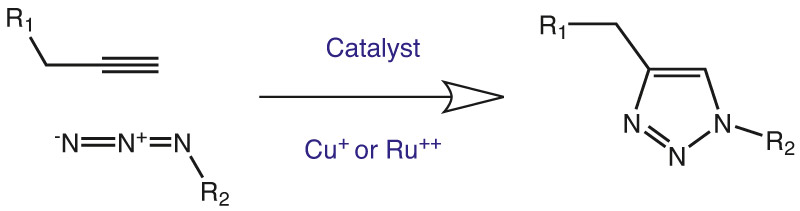
Azido and alkyne functions can cyclise by an intramolecular CuI or Cu0 catalyzed azide-alkyne 1,3-dipolar cycloaddition (CuAAC). This so-called Click Reaction, developed by K. Barry Sharpless and Morton Meldal, has meanwhile grown to a widely used type of reaction orthogonal to many other types of reactions in different kinds of applications. Both residues R1 and R2 can be used either as conjugation partners or as substrates. Due to its high thermodynamic driving force, usually greater than 20 kcal/mol, the click reaction normally proceeds rapidly to completion and also tends to be highly selective for a single product. A variety of azido and alkyne building blocks is available, where some can be incorporated into biomolecules by recombinant syntheses, in particular by non natural protein translation using the amber-suppression-based orthogonal system or by chemical reactions, for example by solid phase synthesis. Then the conjugation with a second molecule carrying the appropriate other function can be done. Tris(benzyltriazolylmethyl)amine (TBTA; RL-2010; see p. 72) is stabilizing copper(I) towards oxidation in solution by forming a complex and catalyzes effectively quantitative, regioselective Huisgen 1,3-dipolar cycloadditions between alkynes and azides (the so called ‚click‘ cycloaddition reaction), in a variety of aqueous and organic solvents. In the literature, it has been gaining widespread use as a biochemical tool for the tagging of proteins and enzymes.
References: