Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 02/05/2018
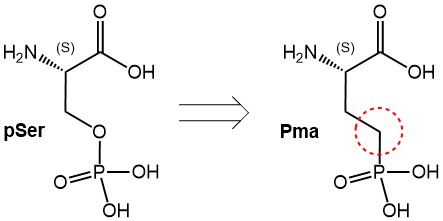
Therefore, the preparation of synthetic phosphorylated peptides is of significant interest for researchers. However, those synthetic efforts are frequently hampered by the lability of the phosphoester bond.
Here we present three phosphono-amino acid derivatives that serve as hydrolysis-stable mimics of pSer, pThr and pTyr, termed Pma (Ser), Pmab (Thr) and Pmp (Tyr). Those derivatives are suitably protected for their use in peptide synthesis using the Fmoc strategy. The tert-butyl protecting groups on the phosphonic acid moieties prevent side reactions by this group and can be removed during final deprotection of the peptide.
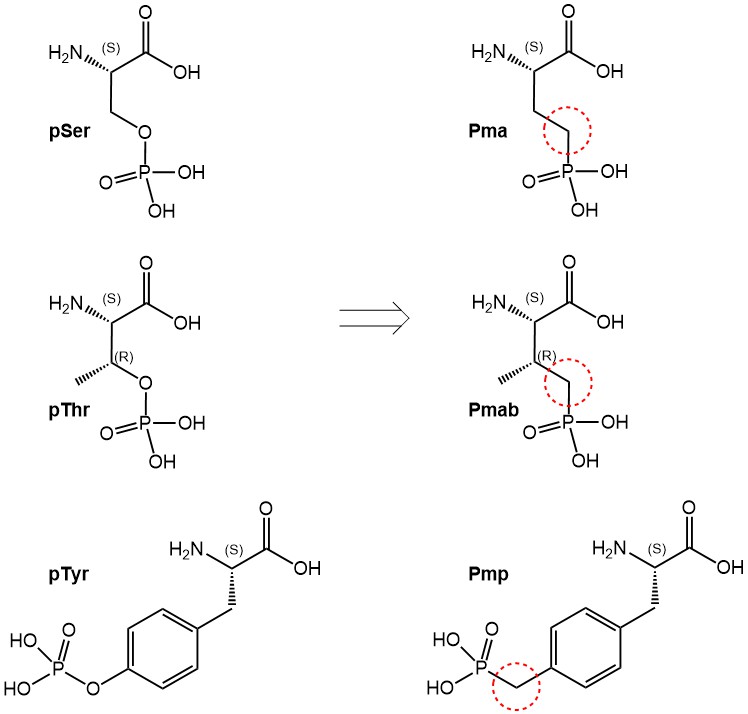
Phosphoserine, -threonine and -tyrosine (left), and their phosphono-analogs Pma, Pmab and Pmp (right).
The stability towards hydrolysis that characterizes the phosphono-derivatives has an additional benefit, which is that cellular phosphatases are unable to remove the phosphate group mimic. Consequently, peptides or semi-synthetic proteins that include Pma, Pmab or Pmp are valuable tools for cell-based experiments.