Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 07/02/2022
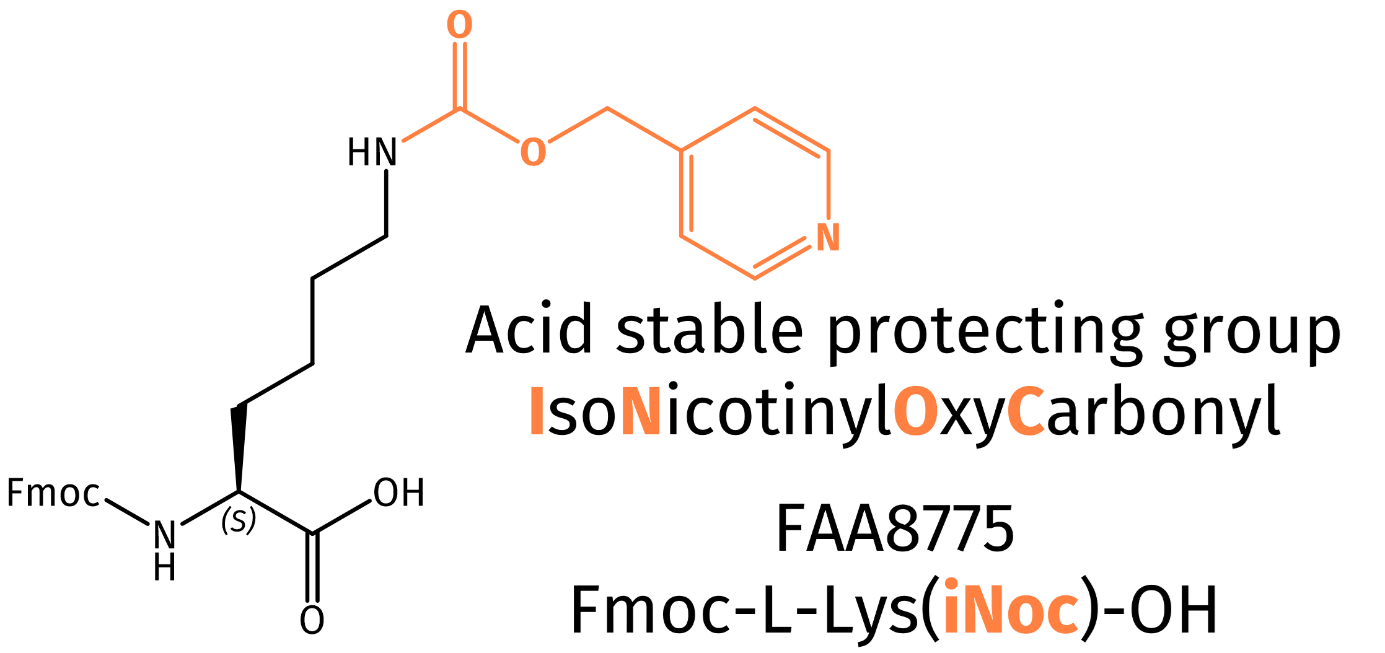
Herein we are presenting isonicotinyloxycarbonyl (iNoc), a protecting group reported in literature for solid-phase peptide synthesis (SPPS) as well as Native Chemical Ligation (NCL). Despite its structural similarity to the benzyloxycarbonyl (Cbz) protecting group, iNoc displays unique properties. The pyridine ring renders the protecting group highly stable towards acidic conditions, while facilitating mild reductive cleavage, e.g. by the action of zinc dust in aqueous acetic acid, by catalytic hydrogenation using 5% Pd/C or by SmI2 in aqueous DMF, which can be safely applied in the presence of almost all functional groups commonly encountered in peptide synthesis. As further benefit, iNoc protected peptides show increased solubility in polar solvents and thus help to prevent aggregation, especially during the synthesis of difficult or long peptides.
In case of benzyloxycarbonyl (Cbz) as epsilon-amino protecting group of lysine, undesired loss may be observed when treating the peptide with acid, e.g. when removing a Boc protecting group or upon cleavage of the peptide from the solid support. The iNoc group assures absolute stability when Boc is cleaved with acid and conversely, it can be removed without affecting the Boc group.
Besides the mild reductive cleavage, Wirth et al. investigated an electroreductive method for the removal of the isonicotinyloxycarbonyl group from hydroxy, thiol, and amino groups. Their electrochemical microreactor allows the rapid and site-selective deprotection of O- (carbonates) or S-iNoc (thiocarbonates) groups without removal of N-iNoc (carbamates).
In summary, the iNoc group provides the following benefits:
Iris Biotech offers a N-alpha-Fmoc N-epsilon-iNoc protected Lysine (FAA8775). Looking for another iNoc protected amino acid? Get in contact in inquire for a custom synthesis of your derivative of choice.
References:
Isonicotinyloxycarbonyl a novel amino protecting group for peptide synthesis; D. F. Veber, W. J. Paleveda, Y. C. Lee, R. Hirschmann; J. Org. Chem. 1977; 42(20): 3286-3288. https://doi.org/10.1021/jo00440a018.
Total Synthesis and Structural Characterization of Caveolin-1; H. Hojo, T. Takei, Y. Asahina, N. Okumura, T. Takao, M. So, I. Suetake, T. Sato, A. Kawamoto, Y. Hirabayashi; Angew. Chem. Int. Ed. 2021; 60(25): 13900-13905. https://doi.org/10.1002/anie.202100826.
Chemoenzymatic Synthesis of the Immunoglobulin Domain of Tim-3 Carrying a Complex-Type N-Glycan by Using a One-pot Ligation; Y. Asahina, S. Kamitori, T. Takao, N. Nishi, H. Hojo; Angew. Chem. Int. Ed. 2013; 52(37): 9733-9737. https://doi.org/10.1002/anie.201303073.
Rapid Electrochemical Deprotection of the Isonicotinyloxycarbonyl Group from Carbonates and Thiocarbonates in a Microfluidic Reactor; K. Arai, T. Wirth; Org. Process Res. Dev. 2014; 18(11): 1377-1381. https://doi.org/10.1021/op500155f.