Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 05/10/2022

Developed by Merrifield in the early 1960s, the solid-phase peptide synthesis (SPPS) is still the method of choice for the preparation of peptides. As peptides are gaining more and more interest, be it for the development of drugs or for innovative materials, the whole peptide market is flourishing. As a result, the SPPS technology itself, but also related reagents and building blocks have been fine-tuned over the years by many research groups.
Typically, in SPPS, the molecule being synthesized is attached to an insoluble support, while reagents and building blocks are added to the suspension. Repetitive cycles of deprotection and coupling allow to grow the peptide chain.
Importantly, in 1977, Barany and Merrifield described the concept of “orthogonality”, which describes the chemoselective removal of protecting groups in the presence of one another without affecting/removing each other when applying defined conditions.
As the diversity of peptides is becoming more and more complex, a high level of orthogonality of the different protecting groups is required for successful synthesis. The scheme below shows an example for tetra-orthogonal protecting groups and their removal conditions.
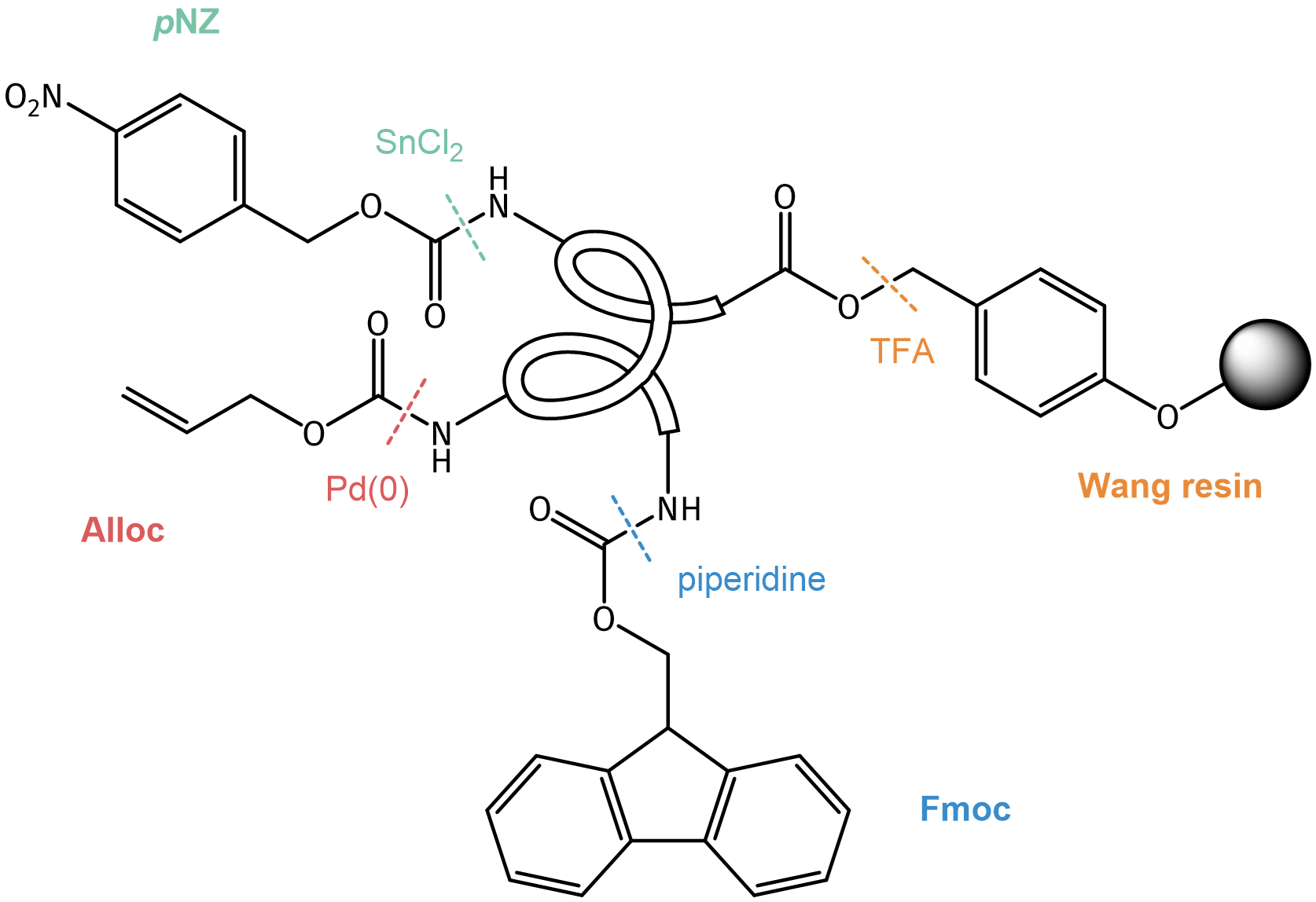
Example for a growing peptide chain with tetra-orthogonal protecting groups.
In general, protecting groups (PGs) used for SPPS can be divided into three categories:
(I) Temporary PGs are removed after each synthetic step.
(II) Permanent PGs are stable to all synthetic manipulations and are removed at the end of the synthesis.
(III) Semipermanent PGs are stable during chain elongation and are removed in the presence of the permanent protecting groups.
A fourth category can be added by so-called “safety-catch protecting groups”. Per definition, such PGs are stable/”safe” to a particular set of conditions until the group is (photo-)chemically converted to its labile form allowing its cleavage.
As an example, herein, we are highlighting the 4,4’-bis(methylsulfinyl)-benzhydryl (Msbh) PG. Msbh is stable to conditions applied in both Boc/Fmoc and Allyl chemistry until its electron-withdrawing sulfoxide groups are reduced. The formed sulfide renders the protecting group acid-labile and thus facilitates the deprotection using TFA.
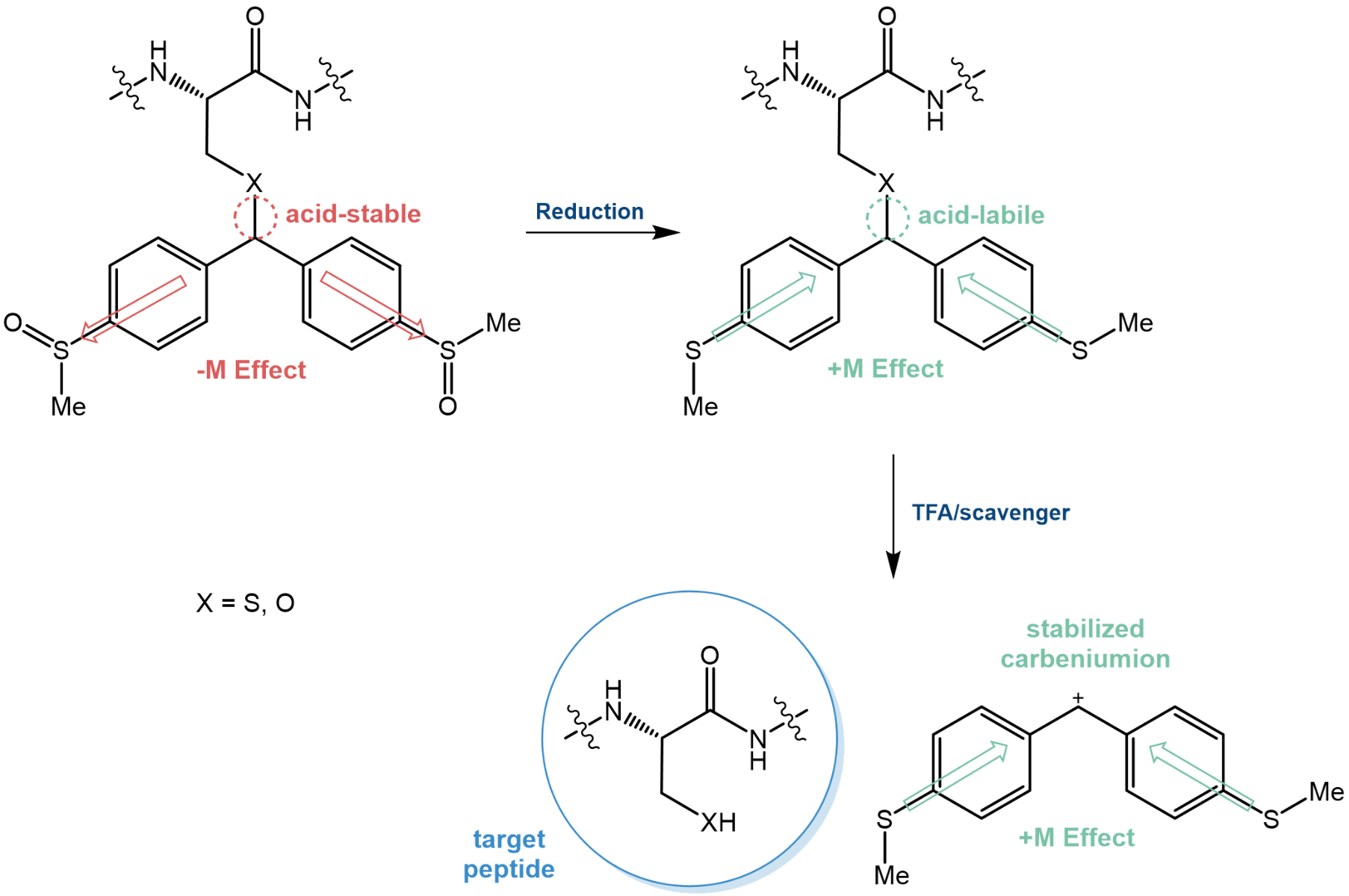
The Msbh safety-catch protecting group.
In the case of cysteine, the Msbh is stable to the deprotection conditions of most common cysteine PGs such as Mmt, Trt, Acm, or Phacm. Another suitable application for Msbh-protected cysteine is whenever there is an odd number of cysteines present in a peptide, where all cysteines except one are disulfide-brigded. In such cases, Msbh can be utilized to protect the side chain of the one cysteine that is supposed to remain a free thiol and is therefore deprotected after all disulfide bonds have been installed. The advantage of using Msbh is that it may greatly reduce the risk of disulfide shuffling during deprotection of the last cysteine residue. Within our portfolio, we offer Fmoc-L-Cys(Msbh)-OH (FAA4155) as well as Fmoc-D-Cys(Msbh)-OH (FAA8150).
To further expand the toolbox of the Msbh safety catch technology, we herein present Fmoc-Ser(Msbh)-OH as building block available in our webshop as D- (FAA8837) and L-isomer (FAA8835). The removal of the PG can be carried out in two steps, first, the reduction with Me3SiCl-Ph3P in THF followed by acidolysis with TFA.
For more information, please see our recent publication together with Prof. Fernando Albericio, in which three safety-catch sulfinyl systems, namely 4-(methylsulfinyl)benzyl (Msib), 2-methoxy-4-methylsulfinylbenzyl (Mmsb), and 4,4′-bis(methylsulfinyl)-benzhydryl (Msbh) are investigated as PGs for six amino acids (Lys, Tyr, Asp, Glu, Ser, and Thr) in combination with Fmoc for α-amino protection. Therefore, a series of Leu−enkephalin amide (H−Tyr−Xxx−Gly−Phe−Leu−NH2) analogs were prepared, whereby Xxx = Lys(Msz), Tyr-(Msbh), Tyr(Msib), Ser(Msbh), Thr(Msbh), Glu(Msib), Glu(Msbh), Asp(Msib), and Asp(Msbh).
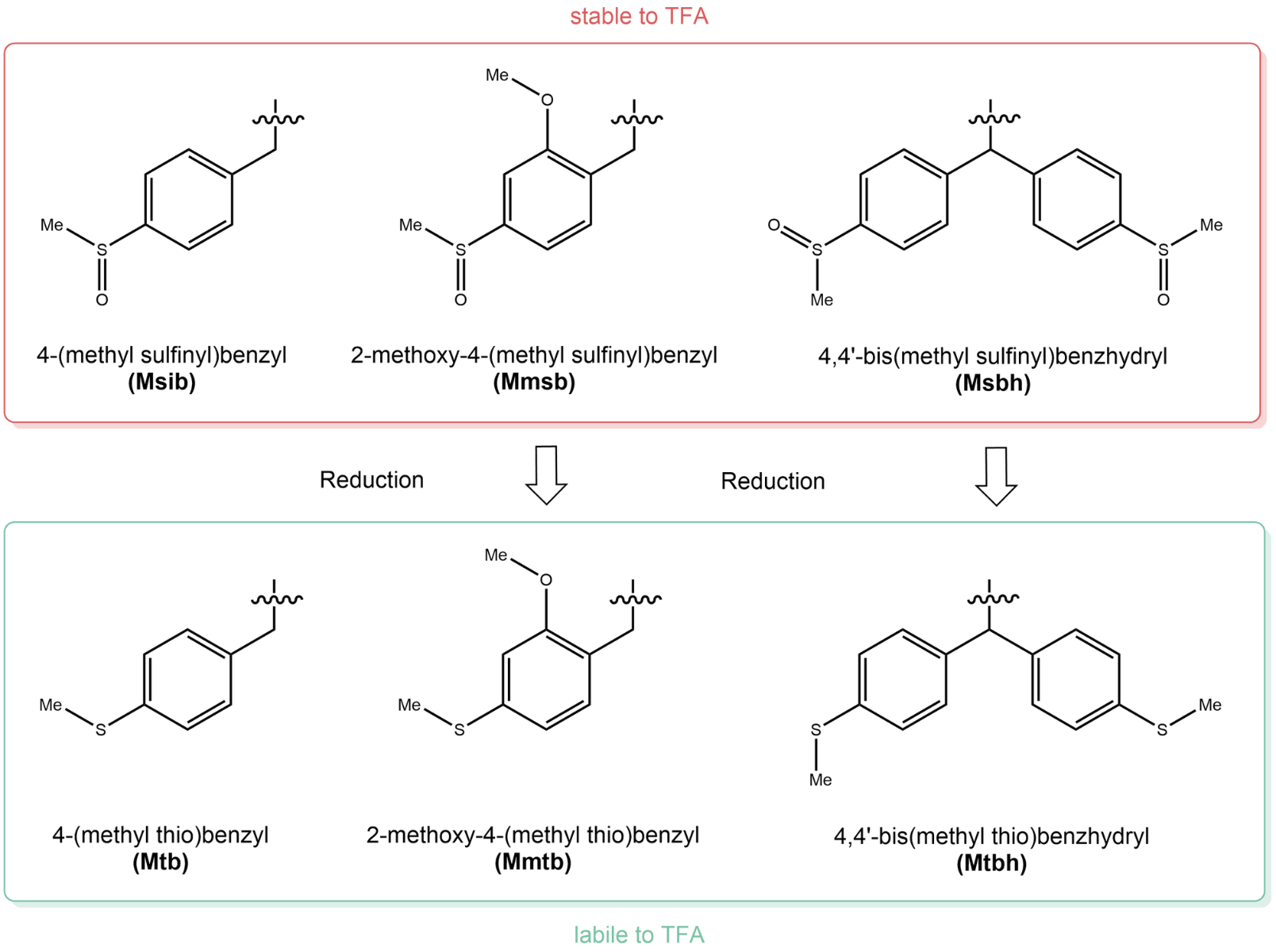
The three sulfinyl-based safety-catch protecting groups Msib, Mmsb and Msbh (J. Org. Chem. 2022; 87: 9443-9453).
➔ Interested in other safety-catch derivatives? Inquire for a custom synthesis!
➔ Dealing with cyclic peptides? Download our brochure for a comprehensive overview of technologies and available Cys protecting groups!
References:
Solid-Phase Peptide Synthesis Using a Four-Dimensional (Safety-Catch) Protecting Group Scheme; S. Noki, E. Brasil. H. Zhang, T. Bruckdorfer, B. G. de la Torre, F. Albericio; J. Org. Chem. 2022; 87: 9443-9453. https://doi.org/10.1021/acs.joc.2c01056
Total Synthesis of Human Hepcidin through Regioselective Disulfide-Bond Formation by using the Safety-Catch Cysteine Protecting Group 4,4'-Dimethylsulfinylbenzhydryl; Z. Dekan, M. Mobli, M. W. Pennington, E. Fung, E. Nemeth, P. F. Alewood; Angew. Chem. Int. Ed. 2014; 53: 2931-2934. https://doi.org/10.1002/anie.201310103
A new safety-catch protecting group and linker for solid-phase synthesis; S. Thennarasu, C.-F. Liu; Tetrahedron Lett. 2010; 51(24): 3218-3220. https://doi.org/10.1016/j.tetlet.2010.04.047
A Reductive Acidolysis Final Deprotection Strategy in Solid Phase Peptide Synthesis Based on Safety-Catch Protection; T. Kimura, T. Fukui, S. Tanaka, K. Akaji, Y. Kiso; Chem. Pharm. Bull. 1997; 45: 18-26. https://doi.org/10.1248/cpb.45.18
Safety-catch anchoring linkage for synthesis of peptide amides by Boc/Fmoc strategy; M. Pátek, M. Lebl; Tetrahedron Lett. 1991; 32(31): 3891-3894. https://doi.org/10.1016/S0040-4039(00)79406-8
The p-(methylsulfinyl)benzyl group: a trifluoroacetic acid (TFA)-stable carboxyl-protecting group readily convertible to a TFA-labile group; J. M. Samanen, E. Brandeis; J. Org. Chem. 1988; 53(3): 561-569. https://doi.org/10.1021/jo00238a016