Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 29/09/2020
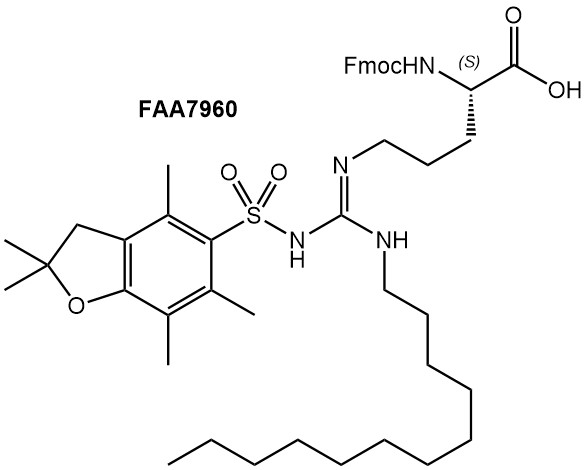
Arginine is characterized by its amphiphilic side chain, with a C3 alkyl chain terminated by a positively charged guanidino group. Since the latter can undergo hydrogen bonding as well as ionic interactions with binding partners, Arginine residues are frequently involved in cellular recognition processes. Consequently, derivatives of Arginine are highly sought after, be it for the introduction of Arg-mimetics to improve pharmacokinetic properties of therapeutic peptides, or to introduce an Arg derivative suitable for site-selective modification.
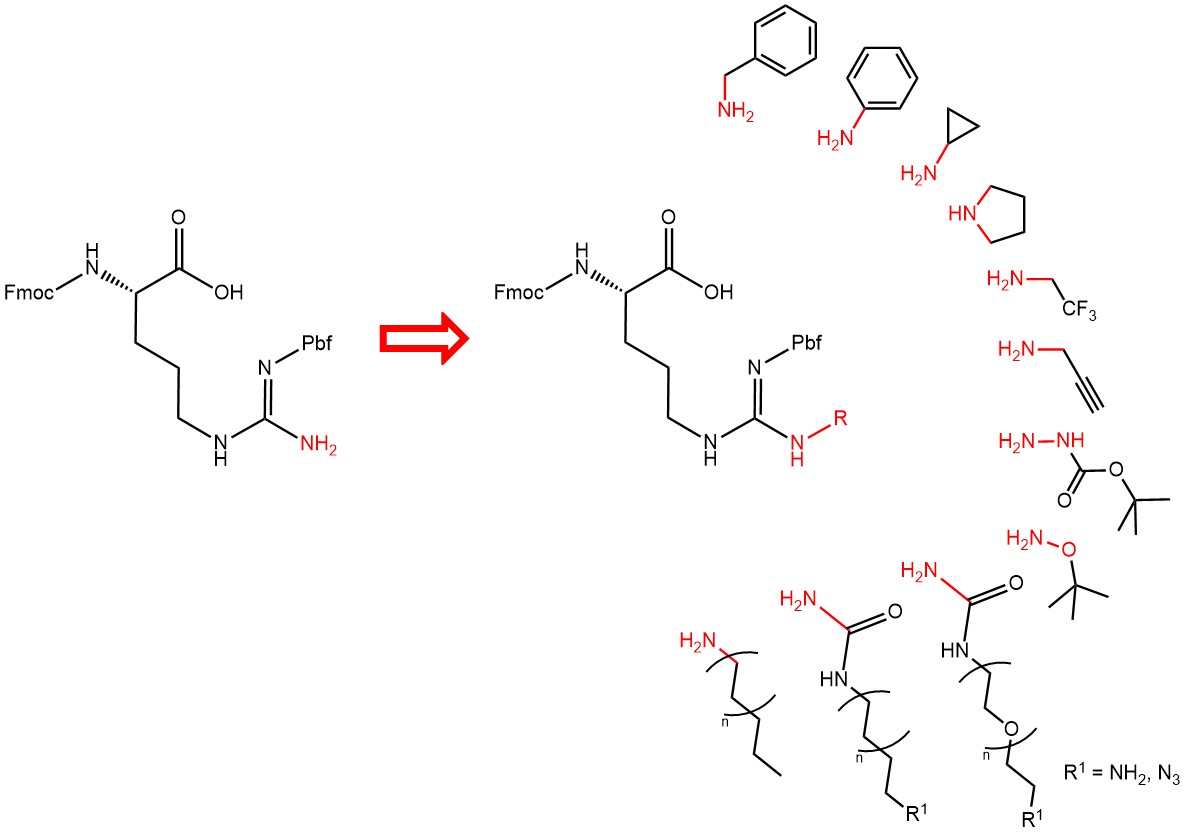
Selection of derivatives of Arginine accessible through our synthetic approach.
Our arginine platform allows us to design derivatives bearing substitutions on one guanidino nitrogen based on virtually any commercially available amine. A process is available to provide Fmoc- and Pbf-protected derivatives, which can instantly be used in any Fmoc/tBu solid phase peptide synthesis protocol.
➔ Do you require a different Arginine derivative? Do not hesitate to contact our Custom Synthesis Service.
References: