Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 03/12/2013
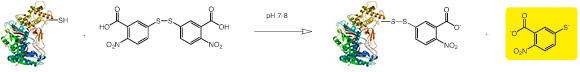
 DTNB, frequently called Ellman’s Reagent, used as reagent for determination of free sulfhydryl groups. Through reaction with aliphatic thiol groups a mixed disulfide of protein thiol and one mole of 2-nitro-5-thiobenzoate per mole of protein sulfhydryl group is being formed. DTNB has little absorbance. Reaction with -SH groups on proteins (from any solvent accessible Cys) under mild alkaline conditions (pH 7-8) produces the 2-nitro-5-thiobenzoate anion, which gives an intense yellow color with an absorption maximum at 409.5 nm (Extinction coefficient: 14150 M -1*cm -1). Ellman’s reagent is sensitive to various buffer ions, therefore, the extinction coefficient used to calculate the number of sulfhydryl groups must be matched to the reaction conditions. In case the thiol groups are in disulfide bonds, they must be reduced under anaerobic conditions prior to reaction with DTNB.
DTNB, frequently called Ellman’s Reagent, used as reagent for determination of free sulfhydryl groups. Through reaction with aliphatic thiol groups a mixed disulfide of protein thiol and one mole of 2-nitro-5-thiobenzoate per mole of protein sulfhydryl group is being formed. DTNB has little absorbance. Reaction with -SH groups on proteins (from any solvent accessible Cys) under mild alkaline conditions (pH 7-8) produces the 2-nitro-5-thiobenzoate anion, which gives an intense yellow color with an absorption maximum at 409.5 nm (Extinction coefficient: 14150 M -1*cm -1). Ellman’s reagent is sensitive to various buffer ions, therefore, the extinction coefficient used to calculate the number of sulfhydryl groups must be matched to the reaction conditions. In case the thiol groups are in disulfide bonds, they must be reduced under anaerobic conditions prior to reaction with DTNB.
Solubility 8 mg/mL in EtOH. Stock solutions c an be prepared in 0.1 M phosphate buffer, which are stable over several weeks.
References:
|