Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorChemical name: N-alpha-(9-Fluorenylmethyloxycarbonyl)-N-epsilon-(o-nitroveratryloxycarbonyl)-L-lysine // Synonyms: Fmoc-Lys(Nvoc)-OH, (S)-2-(((9H-fluoren-9-yl)methoxy)carbonylamino)-6-((4,5-dimethoxy-2-nitrobenzyloxy)carbonylamino)hexanoic acid
Starting at $325.00
2-nitroveratryl (oNv) is a new orthogonal group which is compatible with SPPS protocols and that can be cleaved by photolysis (350 nm) under ambient conditions. See also our product Fmoc-L-Cys(oNv)-OH (FAA3970), where o-nitroveratryl has been used for thiol protection. In order to demonstrate the versatility of this approach, it was applied to the synthesis of a number of model peptides: oxytocin, alpha-conotoxin ImI, and human insulin.
Synthesis, stability and optimized photolytic cleavage of 4-methoxy-2-nitrobenzyl backbone-protected peptides. Erik C. B. Johnson and Stephen B. H. Kent; Chem. Commun. 2006; 1557-1559. DOI: 10.1039/b600304d
Photomodulation of Protein Trans-Splicing Through Backbone Photocaging of the DnaE Split Intein; Luis Berrade, Youngeun Kwon, and Julio A. Camarero; ChemBioChem 2010; 11: 1368 -1372. DOI: 10.1002/cbic.201000157
2-Nitroveratryl as a Photocleavable Thiol-Protecting Group for Directed Disulfide Bond Formation in the Chemical Synthesis of Insulin; Karas J.A., Scanlon D.B., Forbes B.E., Vetter I., Lewis R.J., Gardiner J., Separovic F., Wade J.D., Hossain M.A.; Chem. Eur. J. 2014; 20: 9549-9552. DOI: 10.1002/chem.201403574.
Syntheses and kinetic studies of cyclisation-based self-immolative spacers; Steve Huvelle, Ahmed Alouane, Thomas Le Saux, Ludovic Jullien and Frédéric Schmidt; Org. Biomol. Chem. 2017; 15: 3435-3443. DOI: 10.1039/C7OB00121E
Self-Immolative Spacers: Kinetic Aspects, Structure-Property Relationships, and Applications; Ahmed Alouane, Raphaël Labruère, Thomas Le Saux, Frédéric Schmidt, and Ludovic Jullien; Angew. Chem. Int. Ed. 2015; 54: 7492-7509. DOI: 10.1002/anie.201500088
Fluorogenic Label for Biomolecular Imaging; Luke D. Lavis, Tzu-Yuan Chao, and Ronald T. Raines; ACS Chem. Biol. 2006; 1(4): 252-260. DOI: 10.1021/cb600132m
Prodrug Strategies Based on Intramolecular Cyclization Reactions; Daxian Shan, Michalis G. Nicolaou, Ronald T. Borchardt, and Binghe Wang; J. Pharm. Sci. 1997; 86(7): 765-767. DOI: S0022-3549(97)00069-5
Intramolecular Cyclization Assistance for Fast Degradation of Ornithine-Based Poly(ester amide)s; Caroline de Gracia Lux, Jason Olejniczak, Nadezda Fomina, Mathieu L. Viger, Adah Almutairi; J. Polym. Sci. Part A: Polym. Chem. 2013; 51: 3783–3790. DOI: 10.1002/pola.26788
Design and Synthesis of Photochemically Controllable Restriction Endonuclease BamHI by Manipulating the Salt-Bridge Network in the Dimer Interface; Masayuki Endo, Koji Nakayama, and Tetsuro Majima; J. Org. Chem. 2004; 69: 4292-4298. DOI: 10.1021/jo035774n
Development of photolabile caged analogs of endothelin-1; S. Bourgault, M. Le´tourneau, A. Fournier; Peptides 2007; 28: 1074–1082. doi:10.1016/j.peptides.2007.02.013
Total chemical synthesis of photoactivatable proteins for light-controlled manipulation of antigen–antibody interactions; Shan Tang, Zhengpeng Wan, Yiren Gao, Ji-Shen Zheng, Jing Wang, Yan-Yan Si, Xin Chen, Hai Qi, Lei Liu and Wanli Liu; Chem. Sci. 2016; 7: 1891-1895. DOI: 10.1039/c5sc03404c
Do you need larger quantities for your development or production?
Please send me more information about
Nitrodibenzofuran (NDBF) is a photocleavable side chain protecting group that can be removed by photolysis upon irradiation with UV-light or – es
view details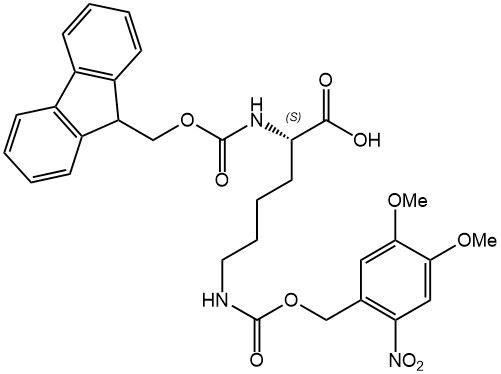
o-Nitroveratryloxycarbonyl (Nvoc) is a photocleavable side chain protecting group for lysine that can be removed by irradiation with UV li
view details






