Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorChemical name: N-alpha (9-Fluorenylmethyloxycarbonyl)-N-alpha-(o-nitroveratryl)-glycine // Synonyms: 2-((((9H-fluoren-9-yl)methoxy)carbonyl)(4,5-dimethoxy-2-nitrobenzyl)amino)acetic acid, N-alpha (9-Fluorenylmethyloxycarbonyl)-N-alpha-(4,5-dimethoxy-2-nitrobenzyl)-glycine
Starting at Please inquire
Tag for internal solubilization, which can be cleaved by photolysis (350 nm) under ambient conditions. See also our product Fmoc-L-Cys(oNv)-OH (FAA3970), where o-nitroveratryl has been used for thiol protection.
This compound is a potential building block for the construction of (customized) peptide nucleic acids (PNAs) and for peptoid synthesis.
Synthesis, stability and optimized photolytic cleavage of 4-methoxy-2-nitrobenzyl backbone-protected peptides. Erik C. B. Johnson and Stephen B. H. Kent; Chem. Commun. 2006;
1557-1559. DOI: 10.1039/b600304d
Photomodulation of Protein Trans-Splicing Through Backbone Photocaging of the DnaE Split Intein; Luis Berrade, Youngeun Kwon, and Julio A. Camarero; ChemBioChem 2010; 11: 1368 -
1372. DOI: 10.1002/cbic.201000157
Karas J.A., Scanlon D.B., Forbes B.E., Vetter I., Lewis R.J., Gardiner J., Separovic F., Wade J.D., Hossain M.A.; 2-Nitroveratryl as a Photocleavable Thiol-Protecting Group for
Directed Disulfide Bond Formation in the Chemical Synthesis of Insulin; Chem. Eur. J. 2014; 20: 9549-9552. DOI: 10.1002/chem.201403574.
Do you need larger quantities for your development or production?
Please send me more information about
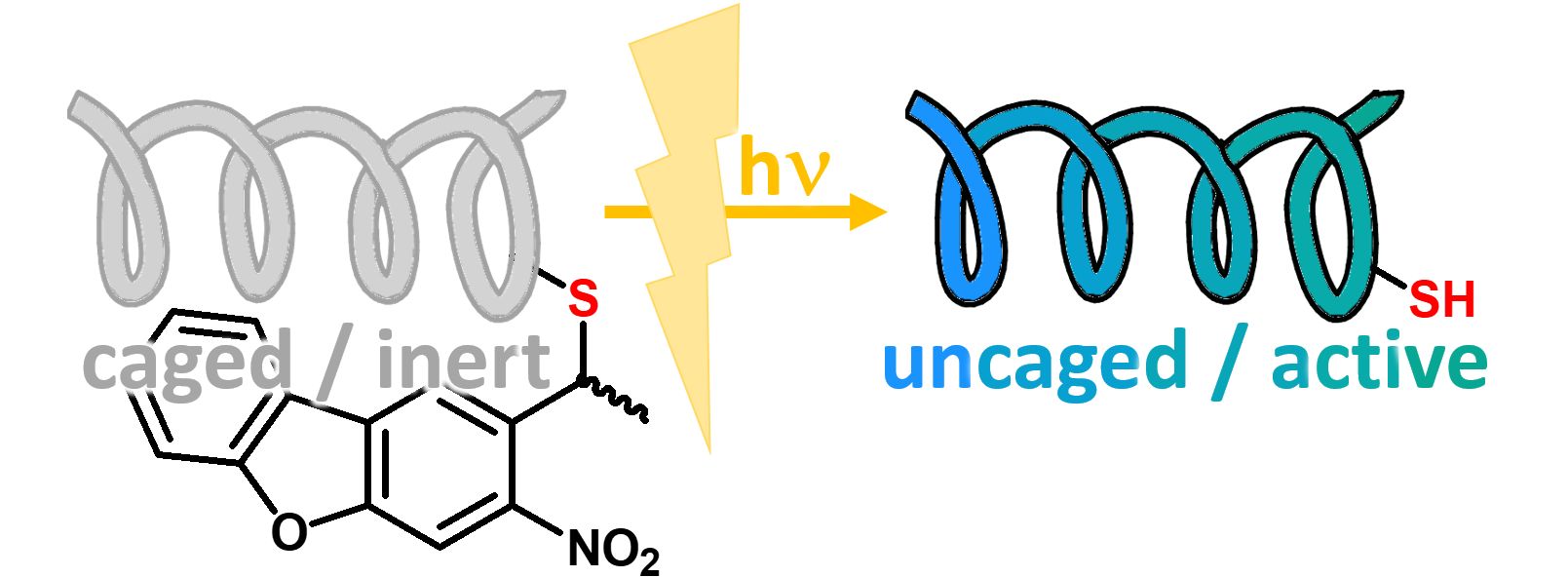
Nitrodibenzofuran (NDBF) is a photocleavable side chain protecting group that can be removed by photolysis upon irradiation with UV-light or – es
view details






