Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
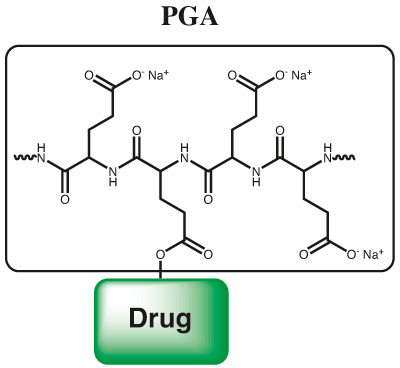
Continue to Iris Biotech GmbHSend request to US distributorPolyglutamates are well known to be highly biocompatible, biodegradable and multifunctional polymers, which have already been used as building blocks in polymer drug conjugates and polymeric micelles. Those systems have been utilized for various medical applications ranging from therapy to molecular imaging. Furthermore, a PGA paclitaxel conjugate has already entered clinical studies: Opaxio™ PGA-paclitaxel (PTX) conjugate is currently in
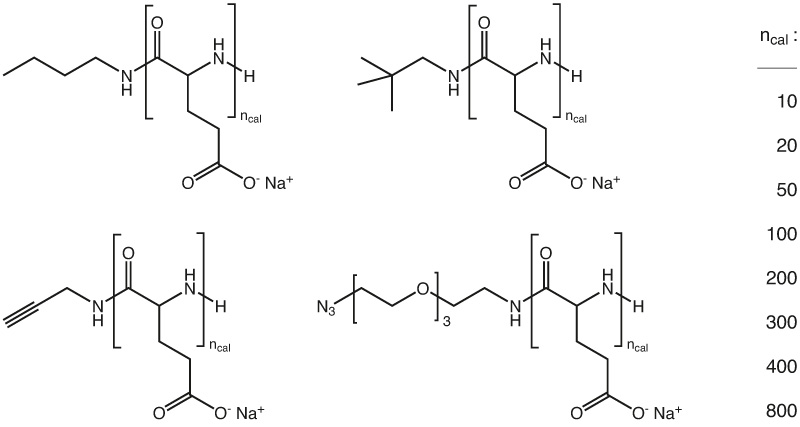
phase III of clinical trials as maintenance therapy in ovarian cancer and has been granted orphan drug designation by the FDA for the treatment of malignant brain cancer. In this context, a synthetic pathway to a plethora of functional polyglutamates (homopolymers, block-co-polymers) with well-defined structure, adjustable molecular weight (MW) and low dispersity (D = Mw/Mn < 1.2) applying the ring opening polymerization (ROP) of N-carboxyanhydrides (NCA) are offered. Additionally, as the acid moieties of the polyglutamates can be activated, various functionalities were introduced by “post-polymerization modification” yielding a set of orthogonal reactive side chains. The reactive moieties, such as azides, maleimides, thiols, or alkynes offer the opportunity of specific conjugation of drugs, targeting moieties or markers.
Besides introducing reactive groups, the functionalization strategy has also been used for PEGylation of PGA. This modification could reduce charge induced interactions and therefore change pharmacological properties such as blood circulation.
In summary, a tool kit of various polyglutamates is offered enabling the synthesis of a variety of polymer drug conjugates or polymer based imaging agents. The functional polymeric precursors allow functionalizing and therefore adjusting the polymer properties to many desired applications.
Background information:
An ideal polymer to be used as carrier for drug delivery or molecular imaging should be characterized by
Most polymer conjugates in the market and in the clinics use N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers, PEG or more recently polyglutamic acid (PGA) as carriers. Biopersistent carriers (PEG, HPMA) present disadvantages, if chronical parenteral administration and/or high doses are required as there is the potential to generate ‘lysosomal storage disease’ syndrome. Alternatively, polyglutamates are well known to be highly biocompatible, biodegradable (by thiol protease cathepsin B) and multifunctional polymers, which have been already applied to various applications that range from drug delivery systems, tissue engineering, sensing, and catalysis. PGA is considered a promising material for the design of novel nanomedicine due to its high biocompatibility, multivalency and in vivo degradability. As a prominent example for its use as nanopharmaceutical, one has to mention a conjugate of polyglutamic acid (PGA) and paclitaxel (OpaxioTM, formerly Xyotax, PPX, CT-2103) in phase III of clinical trials. Another clinical example is provided by several polymeric micelles – firstly developed by Kataoka – that were designed based on the block-copolymer PEGPGA, namely NK 105, NK-6004, Nanoplatin or NC-4016 in Phase I-III trials. Other recent examples of the use of this multifunctional, biodegradable polyanionic carrier can be found in many drug delivery applications not only in cancer, but also in other diseases including tissue regeneration. PGA has also been used due to its multivalency in the development of polymer-based combination therapy applications.
Polyglutamates are commonly obtained by ring-opening
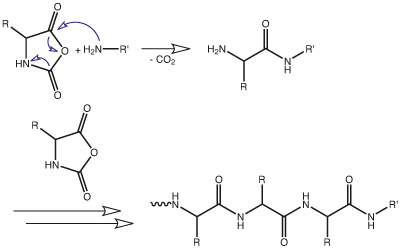
PGA - A Modern Versatile Polymer as Drug Carrier for Drug Delivery, Tissue Engineering, Sensing, Catalysis, NanoMedicine
Poly(glutamic acid) is a biocompatible and biodegradable polymer which can be conjugated through side chain condensation with any suitable molecule. Due to a controlled proprietary and patented process with living polymerization technology a superior quality of PGA is achieved. Usual poly amino acids carry significant amounts of cyclic structures, carbamates or isocyanates. Through a very well controlled polymerization process, well defined terminal groups and polymeric structures are achieved in high purity and with superior polydispersity. Through “living polymer” technology also multifunctional PGA polymers can be produced through post polymerization modification. PGA can be used for polymer therapeutics application for large biopharmaceuticals and also for small molecule drugs. A controlled loading of small molecules onto PGA polymer can be achieved and brings the advantage of polymer therapy also to small molecules.
Published Applications:
In the following published application, PGA equips a hydrophobic small peptoidic drug molecule which has poor water solubility with superior pharmacokinetic properties, excellent water solubility, and increased membrane permeability.
Reference:
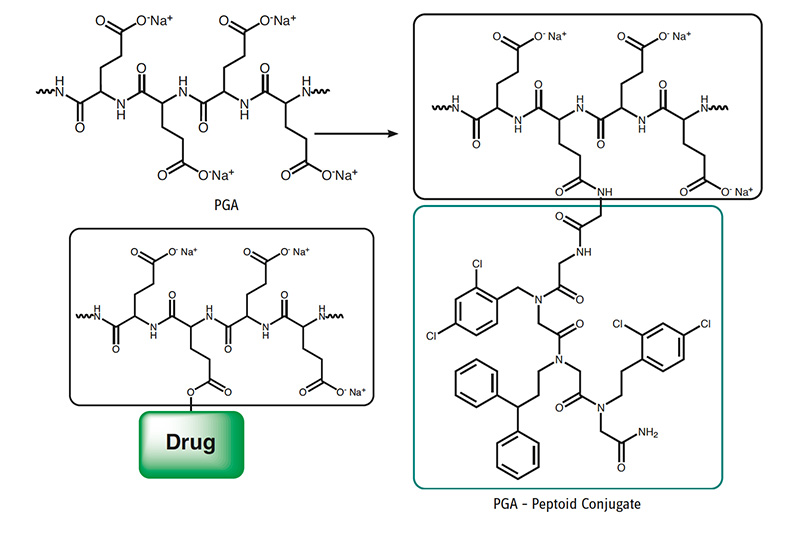
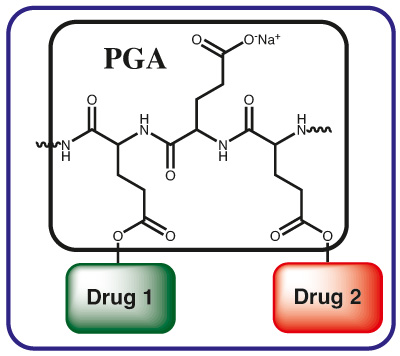
Drug Carrier and Release System for Multiple Drug Therapy
Through post polymerization modification, PGA can be equipped with additional functional groups, like alkyne or azides for click conjugation; however, also the base polymer can be loaded with different molecules as shown in the following example. Paclitaxel (PTX) is a widely-used potent cytotoxic drug that also exhibits anti-angiogenic effects at low doses. Its use at its full potential is limited by severe side effects. The PGA polymer PTX nano-scaled conjugate passively targets tumor tissue exploiting enhanced permeability and retention effect. The polymer is enzymatically-degradable, leading to PTX release under lysosomal acidic pH. The cyclic RGD peptide enhances the effect of PGA-PTX alone by targeting αvβ3 integrin, which is overexpressed on tumor endothelial and epithel cells.
Reference:
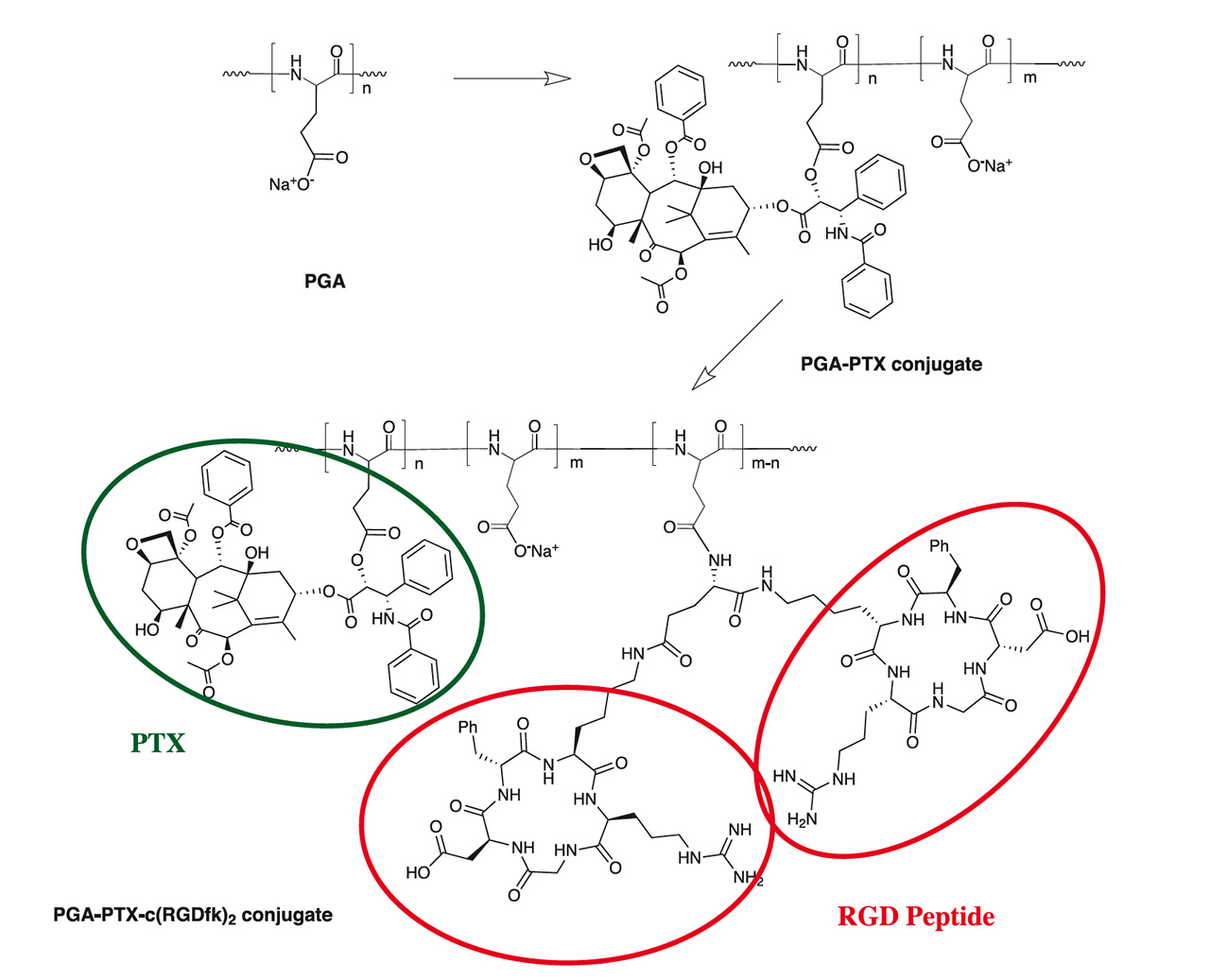
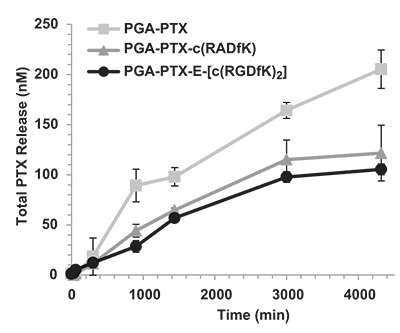
Bi-functional PGA Drug Carrier available for Sophisticated Applications: Combination Therapy – Personalized Medicine
PGA provides ideal possibilities for multi-drug therapies. Already the base PGA polymer can be utilized for these purposes, while multifunctional derivatives increase the number of options for the medicinal chemist.

References: