Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 26/01/2021
The ultimate goal of intracellular drug delivery is the spatial and temporal control of payload release upon cell entry without decomposition during transit. Thus, the interest in selective activation of bioactive agents for advanced therapeutics and diagnostics, aiming to efficiently suppress background interferences and attenuate systemic toxicity, is constantly growing. Over the last years, a new tool for controlled payload transport and release was developed to overcome this barrier. Self-immolative linkers (SILs) are a class of spacers that disintegrate upon a certain trigger to release the conjugated biomolecule in a traceless manner. Typically, the desired drug is conjugated to the proximal end of the spacer, whereas the distal end, which typically initiates the decomposition, is protected, e.g. by being bound to a carrier. Upon deprotection and self-immolation, the conjugated cargo is finally released.
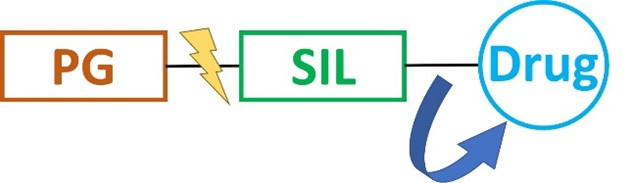
Schematic illustration of the concept of self-immolative linkers. PG = protecting group.
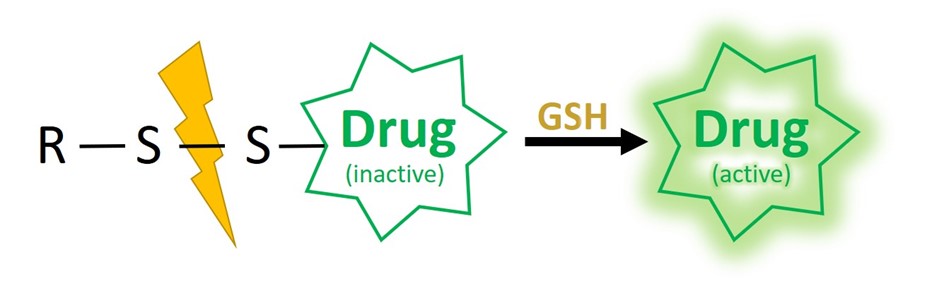
One powerful system relies on disulfide-based self-immolative linkers. The overall red/ox potential in the human blood is oxidative, making disulfide linkages stable during circulation. In contrast, the intracellular milieu of mammalian cells is characterized by an overall reductive potential, thus allowing to revert the disulfide bond formation. Furthermore, cytoplasm typically shows millimolar (1-10 mM) concentrations of the reductant tripeptide glutathione (GSH) in contrast to 2-10 µM in blood and other body fluids.
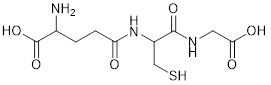
Chemical structure of the tripeptide Glutathione.
Notably, GSH has been recognized as a biomarker of tumor development. Elevated levels are reported inside tumor mass (~100-fold) and cancer cells (~sevenfold) compared to those of normal tissues and cells, respectively. This drastic discrepancy in GSH concentration renders it possible to develop GSH-responsive materials which can be selectively activated in pathological tissues. Those specifications allow for sufficient stability of the linker in the extracellular milieu but spontaneous self-immolative reaction within the cytosol upon GSH-mediated disulfide cleavage.
SILs can be mainly classified into two groups depending on the self-immolative fragmentation process either based on the quinone methide cascade (e.g. 1,6-elimination) or an intramolecular cyclization reaction. In addition, the linker’s chemical composition (e.g. disulfide ethoxycarbonyl (SSE) vs. disulfide benzyloxycarbonyl (SSB)) results in chemically tunable kinetics of the self-immolative cleavage due to different response rate towards GSH, showing higher rates for SSB-based DSILs compared to SSE-based ones. Thus, the choice of the linker allows for fine-tuning of the cleavage speed and payload release.
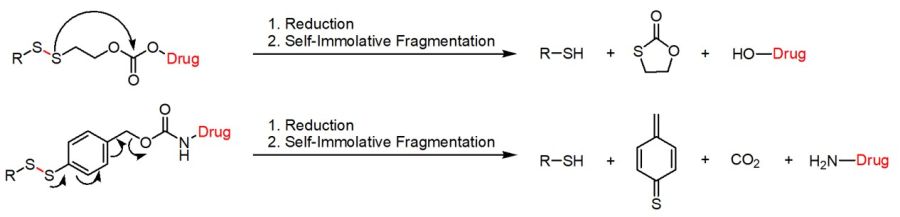
Disulfide-based self-immolative linkers either based on an intramolecular cyclization cascade or an 1,6-elimination.
Please find many different disulfide-based self-immolative linkers within our section Related Products (see below). If you cannot find your desired derivative, please contact us for a custom synthesis!
Interested in further information on linker technologies? -> Please feel free to download our new brochure on Linkerology!
References: