Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 27/10/2020
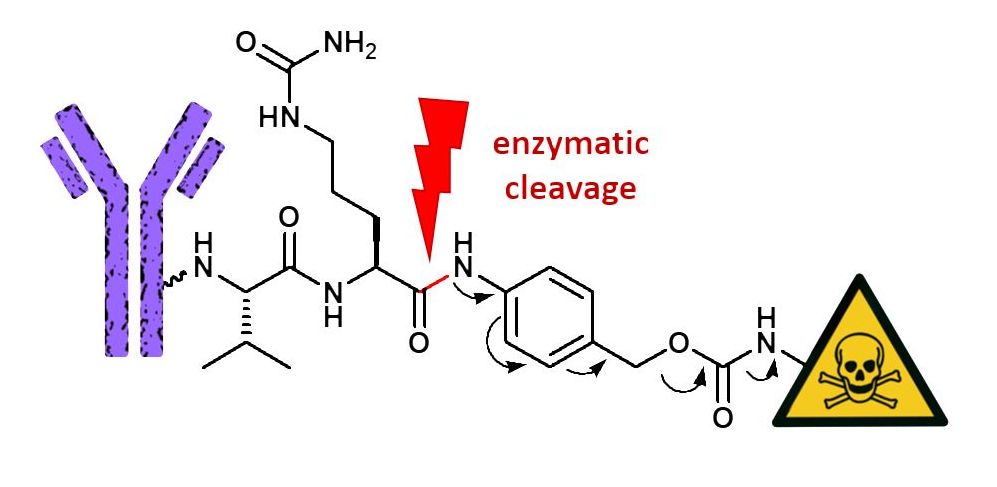
The conjugation of highly potent small drug molecules to target specific biomolecules, like antibodies, is a sophisticated approach, particularly in the field of cancer therapy. Such a synergistic combination allows to circumvent the disadvantages of antibodies, like low potency, as well as the drawbacks of small drug molecules, like low specificity and unwanted side effects.
Linkers, which are implemented to combine both moieties, need to provide sufficient stability during systemic circulation while providing rapid and efficient release of the cytotoxic drug in its active state upon reaching its target.
To fully suit the application’s needs, various linker systems have emerged, including permanent linkers as well as chemically or enzymatically cleavable ones. The latter ones include peptide linkers, which are distinguished by their increased serum stability as well as rapid enzymatic release upon internalization into the target cell. First generation peptide linkers like Gly-Phe-Leu-Gly and Ala-Leu-Ala-Leu were prone to aggregation of the conjugates as well as slow drug release. These limitations are tackled by utilizing dipeptides, such as the Valine-Citrulline motif.
The Val-Cit fragment serves as substrate for cathepsin, a lysosomal protease, which is typically not found extracellularly except under pathological conditions, e.g. in metastatic tumors. Cleavage of the amide bond by hydrolysis is followed by a 1,6-elimination of the para amino benzyl alcohol (PABC) spacer, fragmentation, and traceless release of the drug molecule inside the cell.
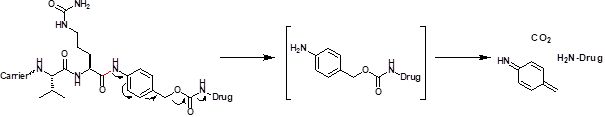
Valyl-citrullyl dipeptide fragment as recognition motif of cathepsin undergoes cleavage by hydrolysis leading to a 1,6-elimination with fragmentation and traceless release of the drug molecule.
This prominent motif was utilized by Seattle Genetics for the development of the ADC Adcetris®, which is used for the treatment of Hodgkin’s lymphoma and systemic anaplastic large cell lymphoma.
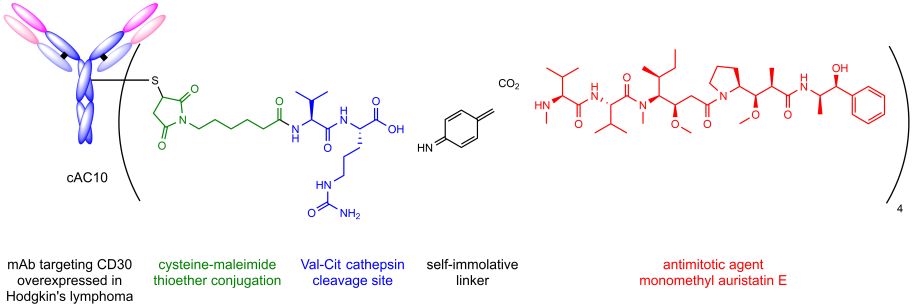
Composition of Adcetris®.
➔ Please click here to see various examples of Val-Cit-based linkers on our Website
➔ Those derivatives are also available based on the Val-Ala motif
Our knowledge and products for linker technologies and ADC design are summarized in our brochure Linkerology, which is available for download free of charge on our Webpage.
To facilitate the development of new ADCs suitable for your desired application, we offer a broad range of protected (Fmoc, Boc) as well as prefunctionalized peptide linkers, e.g. derivatives with a maleimide moiety or bearing an alkyne or azide moiety, which can easily be used for further conjugation via Click reaction.
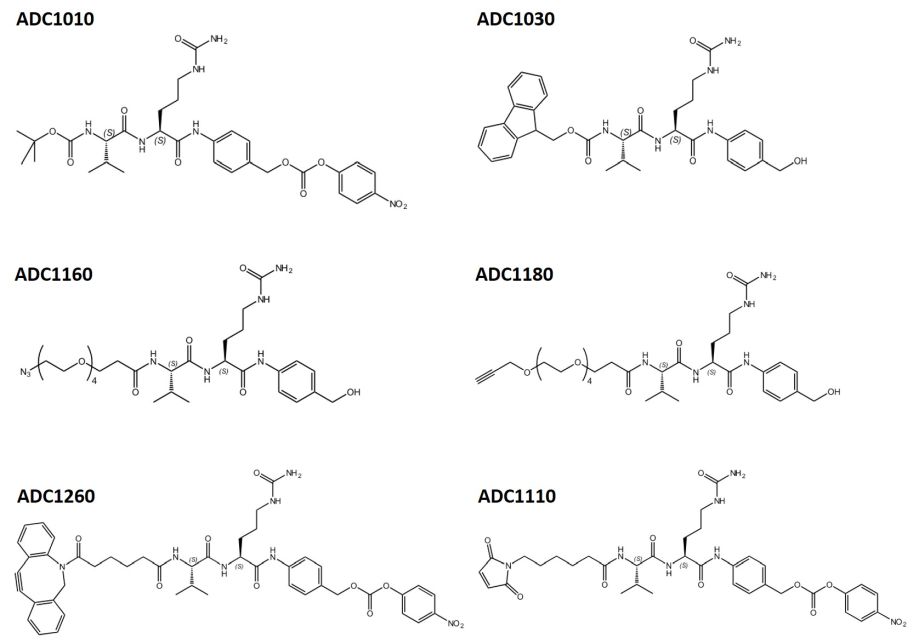
Examples for Vit-Cit-based dipeptide linkers available from Iris Biotech.
➔ You could not find your derivative of choice? Please get in contact for a custom synthesis
References: