Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 29.01.2015
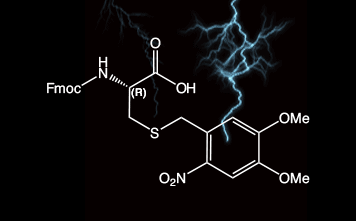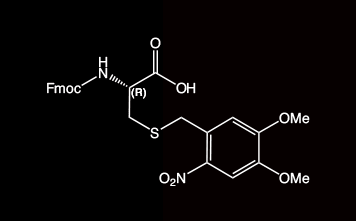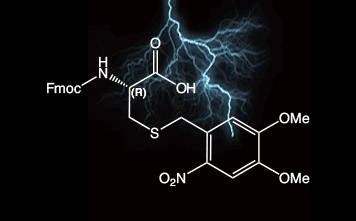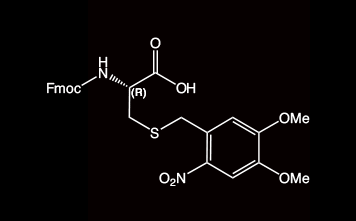
2-Nitroveratryl (oNv) is a new orthogonal group which is compatible with SPPS protocols and which can be cleaved by photolysis (350 nm) under ambient conditions. In combination with complementary S-pyridinesulfenyl activation, disulfide bonds are formed rapidly in situ.
In order to demonstrate the versatility of this approach, it was applied to the synthesis of a number of model peptides: oxytocin, alpha-conotoxin ImI, and human insulin.
This oNV is the latest addition to our repertoire of cysteine protecting groups. Find many more new and innovative technologies for the synthesis of peptides with several disulfide bridges in our brochure “Strategies and Building Blocks for the Synthesis of Cyclic Peptides”.