Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 13.08.2014
Replacement of amide bonds in the backbone of peptides by 1,4- and 1,5-disubstituted 1,2,3-triazole results in an isosteric mimetic. In the meantime several examples have been published where receptor affinity, cell-internalization properties are retained with enhanced proteolytic stability and improved tumor-targeting capabilities. In summary, the 1,2,3-triazole is an effective replacement for a peptide group.
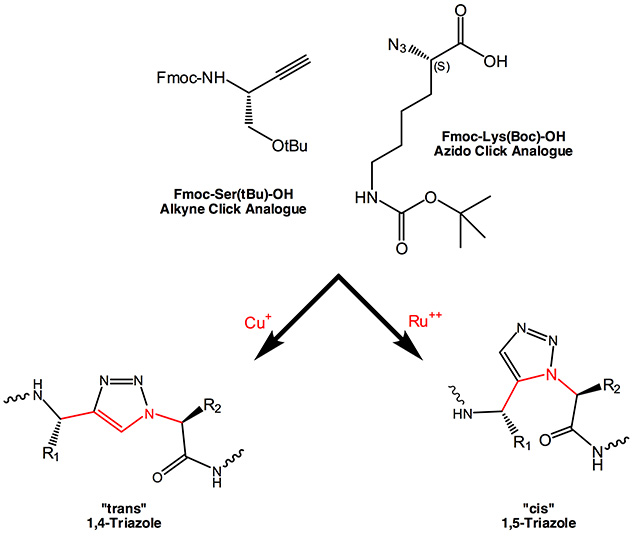
 The Azido function represents the alpha-amino function in native amino acids, while the C-terminus is being replaced by the alkyne moiety. Although in the resulting 1,4-disubstituted 1,2,3-triazole there is one bond more between the two side chain residues, the overall structure of a corresponding dipeptide fragment is being retained and mimics the trans conformation of a peptide bond. The three dimensional structure is maintained, which is confirmed by CD spectra showing the presence of alpha helical structures.
The Azido function represents the alpha-amino function in native amino acids, while the C-terminus is being replaced by the alkyne moiety. Although in the resulting 1,4-disubstituted 1,2,3-triazole there is one bond more between the two side chain residues, the overall structure of a corresponding dipeptide fragment is being retained and mimics the trans conformation of a peptide bond. The three dimensional structure is maintained, which is confirmed by CD spectra showing the presence of alpha helical structures.
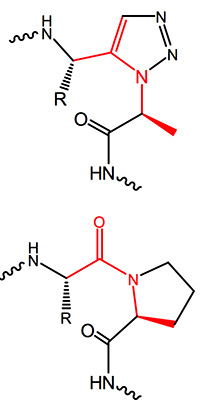
The ratio between cis and trans cyclo addition can to a certain extent be controlled by the appropriate catalyst. Copper catalyzed reactions mainly favour the formation of a 1,4-disubstituted 1,2,3-triazole, the “trans-triazole”, while with bulky chelated Ruthenium often form cis adducts resulting in 1,5-disubstituted 1,2,3-triazoles are gained in high yields. This moiety is totally isosteric to a cis amide bond, mimics and even freezes the appropriate cis bond conformation in proline-containing fragments.
We supply on custom synthesis basis any Alkyne analogue of natural amino acids and will try to meet your requirements for any unusual side chain.

We offer from stock alpha-Azido acids – Azido analogues of Lysine homologues and will try to meet your requirements for any other side chain: