Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 07.02.2017
3.2 Amino-PEG-Acids and Active Esters
PEGylation modification with PEG carboxylic acids or PEG active esters is useful for improving water solubility, increasing chemical stability, decreasing aggregation and toxicity, and reduction of immunogenicity. Amino protected amino-PEG-acids can be used for the PEGylation of solid particles in order to create hydrophilic and reactive surfaces with no non-specific binding issues. The amount of the PEG-NHS ester used in the modification reaction is potentially going to depend on several variables, including:
Protocol for the Conjugation of Active Esters:
a) For aqueous based modifications the reaction can be run in an amine-free buffer at pH 7-8. The PEG NHS ester can be added as a stock solution in a dry organic solvent such as DMAC, DMSO or DMF. This stock solution can be stored for several months frozen at –20°C if care is taken to exclude moisture in the preparation and handling of the NHS ester. The calculated amount of stock is added to the aqueous reactant solution and the reaction will be complete in about 30 minutes to about 2 hours, depending on the specific stoichiometry of the reaction and the desired extent of reaction. The half-life of the NHS ester depends on temperature, pH and structure, i.e. reactivity of the active ester (see table next page). Use your established methods to monitor the reaction. b) For organic based modifications, standard organic procedures may be used, generally adding a solution of the PEG NHS ester to a solution of the reactant containing a tertiary amine, e.g. Hünig’s base, triethylamine or DBU. Homogeneous reactions are typically monitored by TLC or HPLC. Where possible, methylene chloride as the reaction solvent is recommended which also greatly facilitates the work-up. If the solubility of the compound to be modified will not allow this, other appropriate solvents like DMF or DMAC may be used. Optionally, the reaction can be run as slurry and the reaction will draw the reactants into solution, as the incorporated PEG will significantly increase the solubility in many organic solvents, in particular in methylene chloride.
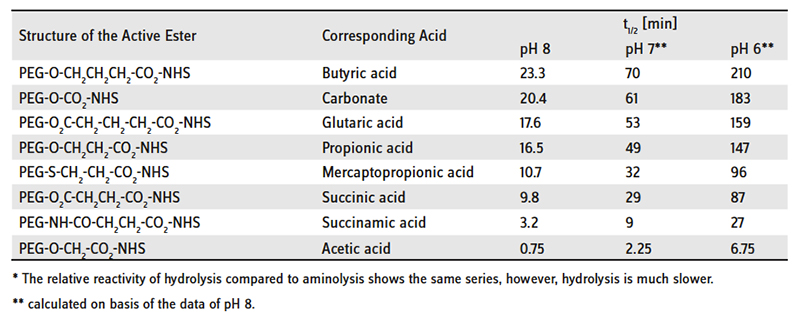
Reactivity of PEG NHS Active Esters towards Aminolysis* at 25°C:
PEG acids can be activated in situ using standard activation methods, e.g., with EDC and NHS in methylene chloride:

Protocol for in-situ Activation to the NHS ester:
Add a methylene chloride solution of the acid to the dry reagents under dry conditions (10-20% molar excess of EDC and NHS in dry methylene chloride, dried over 3A molecular sieves). Stir for several hours or overnight, then evaporate the solvent and use. The reaction mixture can also be treated with a small amount of silica gel to adsorb excess EDC and urea by-product. Filter, then evaporate the solvent and use. NHS should be added together with EDC to prevent formation of an anhydride. DCC and DIC can also be used. Typically use about 1 equivalent and add a solution of the carbodiimide to the acid and NHS (1.1 to 1.2 eq.). PfOH (pentafluorophenol), MSNT (1-(Mesitylene-2-sulfonyl)-3- nitro-1,2,4-triazole), HOCt (Ethyl 1-hydroxy-1H-1,2,3-Triazole- 4-carboxylate), HOPO (2-Hydroxypyridine-N-oxide) and a set of other coupling reagents/leaving groups can be used in place of NHS, if this is of any preference.
Reference: