The presence of catalytic copper, limits the in vivo application of the Click this reaction for several reasons.
We offer custom synthesis of Strained Cyclooctynes and Substituted 1,2,4,5- Tetrazines as building blocks for fast Click reactions in the absence of any catalyst.
Cycloaddition reactions such as the [3+2] azide-alkyne and the [4+2] Diels-Alder reaction, are becoming common conjugation techniques. Applications range from imaging, drug design and development of sensors, thereby spanning the fields of chemical biology, material science, surface and polymer chemistry as well as many other fields.
Introduced in 2002, the copper-catalyzed variant of the azide-alkyne cycloaddition (CuAAC) reaction has found broad applicability in the aforementioned field and as such is currently the most widely used conjugation technique. The presence of copper, however, limits the in vivo application of this reaction for several reasons:
- High cell toxicity
- Undesired oxidation of proteins
- Inhibition of luminescence properties of nanocrystals
To allow fast and efficient in vivo conjugation, new methodologies have emerged that do not require the use of a metal catalysts, while still making use of the bioorthogonal functional groups, i.e. azides and alkynes. The most commonly used approaches can be classified into two categories:
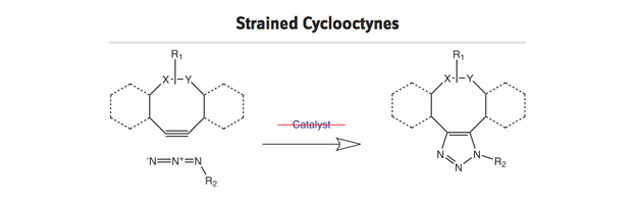
- Strained cyclooctyne systems that react rapidly with azides.
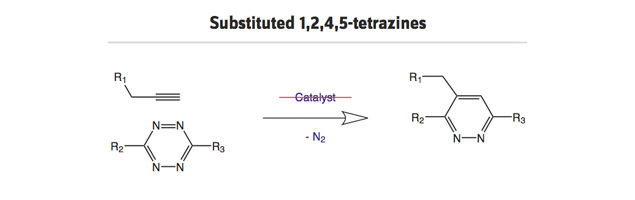
- Substituted 1,2,4,5-tetrazines for fast reactions with (un)strained alkenes/alkynes.
Review articles on copper-free cycloaddition reactions:
- Bioorthogonal labelling of biomolecules: new functional handles and ligation methods; Marjoke F. Debets, Jan C. M. van Hest and Floris P. J. T. Rutjes; Org. Biomol. Chem. 2013; 11: 6439–6455; DOI: 10.1039/c3ob41329b.
- Bioconjugation with Strained Alkenes and Alkynes; Marjoke F. De- bets, Sander S. van Berkel, Jan Dommerholt, A. (Ton) J. Dirks, Floris P. J. T. Rutjes, and Floris L. van Delft; Accounts of Chemical Research 2011; 44(9): 805–815. DOI: 10.1021/ar200059z.
- Azide: A Unique Dipole for Metal-Free Bioorthogonal Ligations; Marjoke F. Debets, Christianus W. J. van der Doelen, Floris P. J. T. Rutjes, and Floris L. van Delft; ChemBioChem 2010; 11: 1168 – 1184. DOI: 10.1002/cbic.201000064.
- Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functio- nality; Ellen M. Sletten, Carolyn R. Bertozzi; Angew. Chem. Int. Ed. 2009; 48(38): 6974–6998. doi: 10.1002/anie.200900942.
- Bioorthogonal chemistry: strategies and recent developments. Ramil CP, Lin Q.; Chem Commun (Camb). 2013; 49(94): 11007-22. doi: 10.1039/c3cc44272a.
- Biomedical Applications of Tetrazine Cycloadditions; Neal K. Deva- raj and Ralph Weissleder; Acc. Chem. Res. 2011; 44(9): 816–827. DOI: 10.1021/ar200037t.
Interested in custom synthesis of strained cyclooctynes or tetrazines?
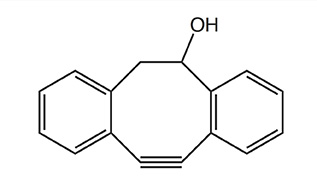
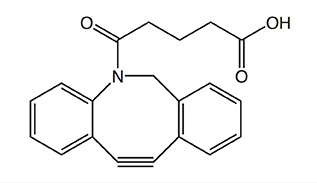
In the table below various strained cyclooctynes are depicted with their related reactivities, as determined by reaction with benzyl azide as model azide. In general, the reactivity increases through the presence of atoms with high electronegativity next to the alkyne function, i.e. good σ-acceptors.
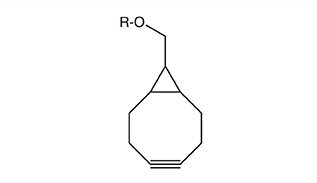
The reactivity will also increase with increasing ring strain, as is the case for dibenzo-fused cyclooctynes (DiBO and DiBAC) and bicyclo[6.1.0]non-4-yne (BCN), whereby the cyclopropane ring is contributing to the additional strain in the latter.
2nd order reaction rate constant k [x 10-3 M-1 s-1]
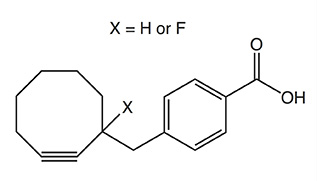 |
1.2 (X = H)
4.3 (X = F)
|
 |
57
|
 |
310
|
 |
110
|
Unlike other cyclooctynes, BCN generates a single regioisomer after reaction with an azide component. It has been shown that BCN also rapidly reacts with other 1,3-dipoles such as nitril oxides and nitrones, as well as substituted 1,2,4,5-tetrazines. In terms of polarity BCN is a highly suitable reactive partner in strain-promoted cycloaddition reactions in aqueous media, as the number of functional groups or phenyl rings is reduced to a minimum, hence allowing efficient bioconjugations in biologically relevant systems.
References of applications with strained cyclooctynes:
- Patent: WO2011136645A1
- Imaging bacterial peptidoglycan with near-infrared fluorogenic azide probes; Peyton Shieh, M. Sloan Siegrist, Andrew J. Cullen, and Carolyn R. Bertozzi; PNAS 2014; 111(15): 5456–5461. DOI: www.pnas. org/cgi/doi/10.1073/pnas.1322727111.
- Readily Accessible Bicyclononynes for Bioorthogonal Labeling and Three-Dimensional Imaging of Living Cells; Jan Dommerholt, Samuel Schmidt, Rinske Temming, Linda J. A. Hendriks, Floris P. J. T. Rutjes, Jan C. M. van Hest, Dirk J. Lefeber, Peter Friedl, and Floris L. van Delft; Angew. Chem. Int. Ed. 2010; 49: 9422 –9425: DOI: 10.1002/ anie.201003761.
- Aza-dibenzocyclooctynes for fast and efficient enzyme PEGylation via copper-free (3+2) cycloaddition; Marjoke F. Debets, Sander S. van Berkel, Sanne Schoffelen, Floris P. J. T. Rutjes, Jan C. M. van Hest and Floris L. van Delft; Chem. Commun. 2010; 46: 97–99; DOI: 10.1039/b917797c.
- Improved synthesis and biological evaluation of chelator-modified α-MSH analogs prepared by copper-free click chemistry; Nicholas J. Baumhover, Molly E. Martin, Sharavathi G. Parameswarapp, Kyle C. Kloepping, M. Sue O’Dorisio, F. Christopher Pigge, Michael K. Schultz; Bioorg. Med. Chem. Lett. 2011; 21(19): 5757 DOI:10.1016/j. bmcl.2011.08.017.
- Synthesis of a DOTA-Biotin Conjugate for Radionuclide Chelation via Cu-Free Click Chemistry; Michael K. Schultz, Sharavathi G. Parameswarappa, and F. Christopher Pigge; Org. Lett. 2010; 12(10): 2398-2401. DOI: 10.1021/ol100774p.
- Dual Labeling of Biomolecules by Using Click Chemistry: A Sequen- tial Approach; Peter Kele, Gabor Mezö, Daniela Achatz, and Otto S. Wolfbeis; Angew. Chem. Int. Ed. 2009; 48: 344 –347. DOI: 10.1002/ anie.200804514.
- Clickable fluorophores for biological labeling—with or without copper; Péter Kele, Xiaohua Li, Martin Link, Krisztina Nagy, András Herner, Krisztián Lörincz, Szabolcs Béni and Otto S. Wolfbeis; Org. Biomol. Chem. 2009; 7: 3486–3490. DOI: 10.1039/b907741c
- Genetic Encoding of Bicyclononynes and trans-Cyclooctenes for Site-Specific Protein Labeling in Vitro and in Live Mammalian Cells via Rapid Fluorogenic Diels−Alder Reactions; Kathrin Lang, Lloyd Davis, Stephen Wallace, Mohan Mahesh, Daniel J. Cox, Melissa L. Blackman, Joseph M. Fox, and Jason W. Chin; J. Am. Chem. Soc. 2012; 134: 10317−10320. DOI: dx.doi.org/10.1021/ja302832g.
- Minimal Tags for Rapid Dual-Color Live-Cell Labeling and Super- Resolution Microscopy; Ivana Nikić, Tilman Plass, Oliver Schraidt, Jędrzej Szymański, John A. G. Briggs, Carsten Schultz, and Edward A. Lemke; Angew. Chem. Int. Ed. 2014; 53: 2245 –2249. DOI: 10.1002/ anie.201309847.
- Moderating Strain without Sacrificing Reactivity: Design of Fast and Tunable Noncatalyzed Alkyne−Azide Cycloadditions via Stereo- electronically Controlled Transition State Stabilization; Brian Gold, Gregory B. Dudley, and Igor V. Alabugin; J. Am. Chem. Soc. 2013; 135: 1558−1569. DOI: dx.doi.org/10.1021/ja3114196.
- Kinetics studies of rapid strain-promoted [3+2] cycloadditions of nitrones with bicyclo[6.1.0]nonyne; Douglas A. MacKenzie and John Paul Pezacki; Can. J. Chem. 2014; 92: 337–340. DOI: dx.doi. org/10.1139/cjc-2013-0577.







