Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 12.08.2014
Beta and gamma homologues of natural amino acids provide a broad use as building blocks for peptidomimetics and the synthesis of heterocyles. Possessing extra carbons between the amino and carboxylic acid function, this class of compounds have greater potential for structural diversity than their alpha-analogues. Even more, these derivatives are in general more stable to hydrolysis or enzymatic degradation compared to natural amino acids.
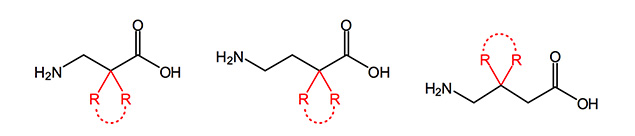
GABA and analogues are involved in several biological functions like cell proliferation and migration, and may play a role in cancer, in mucus overproduction in asthma acting on airway epithelial cells. GABA itself is a neurotransmitter in the central nervous system of mammals and its deficiency is associated with severe neurological disorders, such as Parkinson’s and Huntington’s disease.
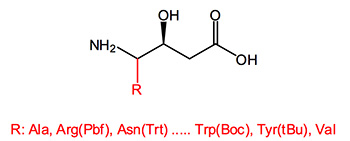 Statine is a gamma amino acid that occurs twice in the sequence of pepstatin, a protease inhibitor that is active against pepsin and other acid proteases, like aspartic acid protease. In this context it has been found that the beta-hydroxy group is important for tight binding and its stereochemistry has a large effect on protease inhibition. A syn diastereomeric position between the amine and hydroxyl groups is essential.
Statine is a gamma amino acid that occurs twice in the sequence of pepstatin, a protease inhibitor that is active against pepsin and other acid proteases, like aspartic acid protease. In this context it has been found that the beta-hydroxy group is important for tight binding and its stereochemistry has a large effect on protease inhibition. A syn diastereomeric position between the amine and hydroxyl groups is essential.
The administration of GABA orally or intravenously is no efficient therapy due to its low lipophilicity, and its poor ability to cross the blood-brain-barrier. Consequently, the design of more lipophilic GABA derivatives capable to cross the BBB, which could inhibit the GABA transaminase, has already been the target of a great number of studies in the past. Already commercially available are derivatives in Vigabatrin® Baclon® Pregabalin®.
Statin is regarded as the key pharmacophore in the renin inhibitor pepstatin isolated from bacteria. Molecules containing these elements inhibit human immunodeficiency virus type 1 (HIV-1) proteinase and other proteases, are active against cathepsin D, virus protease (FIV PR) and are candidates for further drug development in Alzheimer's disease.
GABA, Statine and its analogues are therefore very useful as building blocks with high potential and show that this class of compounds are key structural elements in modern drug design.
Our Tool Box |
Achieved Properties |
|
|