Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 10.11.2020
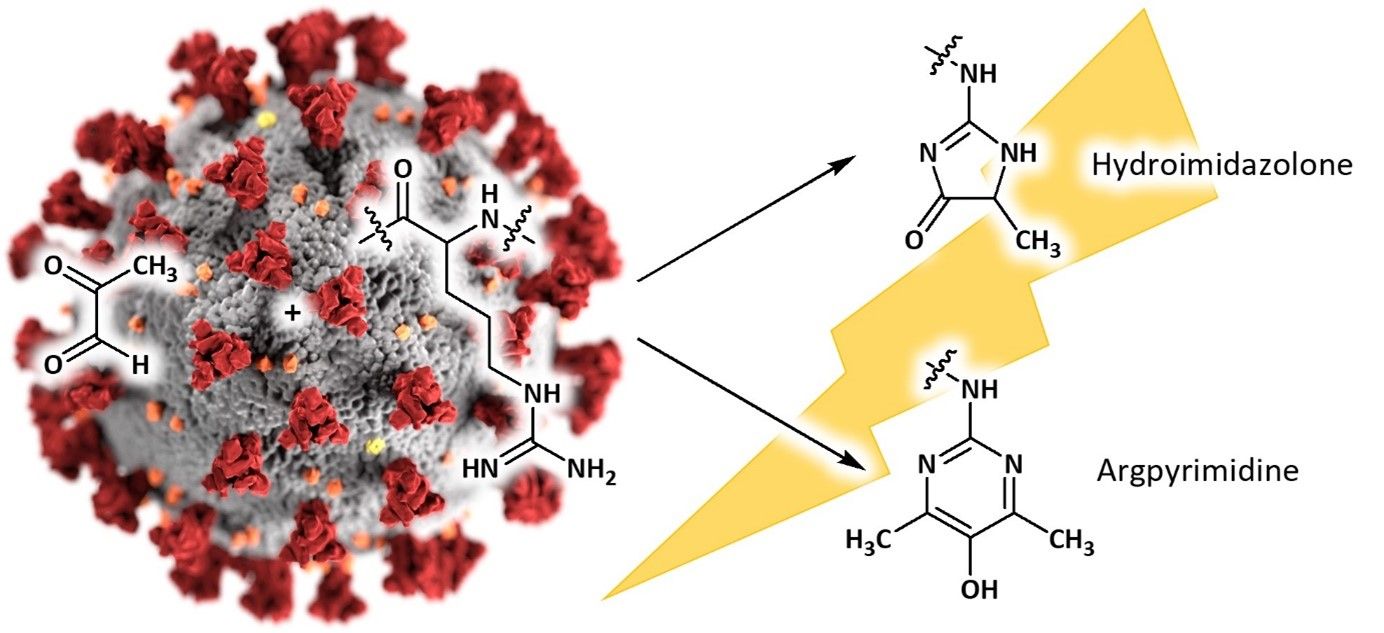
A recent publication highlights protein modification by methylglyoxal (MG) – a dicarbonyl known in the context of the Maillard reaction – as a potential approach to diminish the activity of the SARS-CoV-2 virus. The global pandemic of COVID-19 counts almost 51 million recorded cases including more than one million deaths as of November 11th, 2020 (www.WHO.org). To push the urgent requirement for an anti-viral treatment forward, one approach benefiting from a high degree of investigation is to repurpose well-established drugs and strategies.
One option for improved drug therapy is to increase levels of endogenous reactive metabolites to damage the viral proteome. Among those are reactive oxygen species (ROS), as well as the reactive glycating agent methylglyoxal (MG). ROS can oxidize cysteine residues in proteins to cystine and cysteine sulfenic and sulfonic acids. In the case of MG, mainly arginine residues are targeted and glycated to the hydroimidazolone MG-H1 (HAA3000), resulting in a loss of charge and consequently a loss of all related electrostatic interactions. Typically, this glycation also leads to resistance to proteolytic cleavage close to the site of modification.
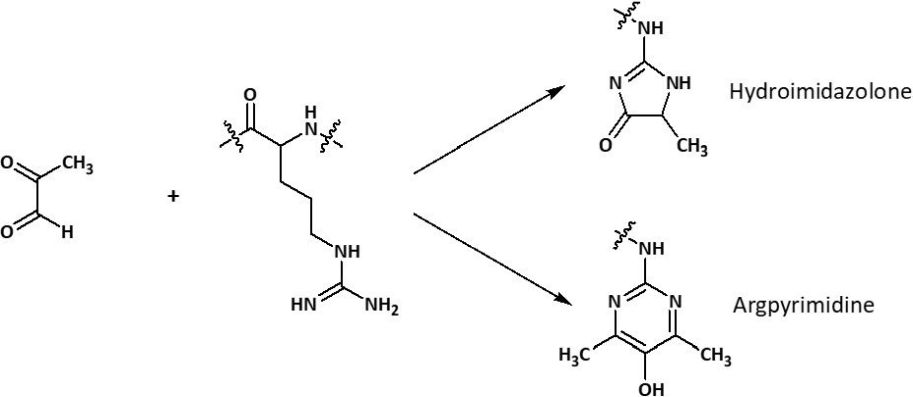
Possible modifications of arginine by methylglyoxal.
The modification of amino acids might lead to changes in a protein’s three-dimensional structure and thus alter its activity or lead to a loss of function. Sequence-based analysis of the SARS-CoV-2 proteome revealed that particularly arginine residues, but not cysteines are accumulated in certain functional domains. This suggests that SARS-CoV-2 is rather resistant to oxidative agents, but sensitive to MG, indicating that modifications of those arginine residues might lead to an inactivation of the virus and virucidal activity. Importantly, studies revealed that crucial arginines are also present in functional domains of the viral spike protein. Thus, dysfunction of the spike protein caused by the modification of arginine residues might block cell fusion and inhibit virion entry, and thus improve the host immune response to the virus.
For your research purposes within this context, Iris Biotech offers a range of methylglyoxal-derived Maillard reaction products, e.g. Argpyrimidine (FAA5530), Glyoxal-hydroimidazolone (HAA2970), Methylglyoxal-hydroimidazolone (HAA3000) and various more (see related products).
References: