Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 17.10.2023

Metal particles are usually not water soluble and nanoparticles of metals and metal oxides tend to agglomerate. Surface modification can prevent particles from sticking together and render their properties to biocompatibility. Improving mechanical stability, enabling or disabling self-organization are further aspects in this context. An interesting and new field is decorating inorganic materials with linkers bearing further reactive functional groups for subsequent attachment of peptides, enzymes or antibodies to facilitate applications like catalysis, chemical sensing, bio-labeling, monitoring of protein-protein interactions, affinity binding, or cell growth trials. In this blog, we focus exemplarily on precious metal and metal oxide nanoparticles.
The number of applications of organosulfur ligands on gold nanoparticles is already very high, as gold is tolerated by organisms and is a very good vehicle for any cargo molecule. Sulfur possesses a high affinity to various metals besides gold, such as silver (Ag), copper (Cu), platinum (Pt), mercury (Hg), or iron (Fe). The metal-sulfur bond is strong enough to immobilize mercapto ligands on the metal surface, however, the monodentate dative thiol-metal bond is not as strong as a true covalent linkage in organic compounds. The chemisorption energy between gold and sulfur is approximately 125 kJ/mol and when sulfur is oxidized to sulfate or sulfonate, the interaction is significantly reduced. Any thiol organic compound may, therefore, leave the surface, if exposed to oxygen in air or solution. It is then also subject to displacement by other thiols or molecules with good nucleophilic donor centers.
Monothiols can be readily removed by reducing agents such as DTT (Cleland’s Reagent) or phosphines like TCEP. To increase the strength of thiol dative bonds, bidental or multivalent ligands can be used. The disulfide lipoic acid (also known as thioctic acid) binds far stronger to metal surfaces and is much more resistant towards removal from the metal surface by DTT and similar reagents than monothiols. An additional benefit is that stench is often operative for monothiols, while lipoic acid derivatives have no odor.

Organic thiols and disulfides form dative bonds on gold surfaces. They can effectively be released with DTT or phosphines like TCEP. A stronger bond is formed with vicinal disulfides like in lipoic acid, which requires reductive activation, e.g. with NaBH4, before it binds very tightly to the metal layer.
To attach a lipoic acid (LA) moiety carrying linker, it has to be reduced to dihydrolipoic acid (DHLA). Both TCEP and NaBH4 can been used for reduction of LA to DHLA. In general, TCEP reduction is carried out in water or aqueous buffer (excluding phosphate, in which TCEP is unstable), in threefold or greater molar excess to the lipoic acid derivative, using an incubation temperature of 25°C to 50°C, for about 1-2 hours. However, each reduction procedure must be optimized for the particular lipoic acid derivative.
Silver nanoparticles are strong light absorbers and light scatterers, and they also display anti-bacterial and anti-viral properties. To render them hydrophilic and water suspendable, they can be coated with a self-assembled PEG monolayer (SAM). PEG linkers impart hydrophilicity, non-antigenicity, and non-immunogenicity to nanoparticles. Carboxy-PEG-lipoamides or -thiols will specifically introduce carboxy functional groups on the surface. A co-coating with methoxycapped PEG thiols creates a water-like monolayer shell bearing a certain number of reactive carboxylic acid functions on the metallic surface. The density of reactive groups can nicely be finetuned by the ratio of methoxy-PEG- to carbyoxy-PEG-thiols. This becomes essential if interaction with large molecules like proteins and antibodies is expected.

Organic thiols form dative bonds to silver surfaces. With a mixture of (a) methoxy end-capped PEG thiols and (b) PEG thiols carrying carboxylic acid functions, a self-assembled monolayer (SAM) can be implemented on the silver surface, where the density of carboxylic acid functions can specifically be designed by the ratio of the different PEG starting materials.
→ Interested in thiol PEG derivatives? Download our brochure PEGylation.
Although some nanomaterials composed of metal oxides have excellent physical and chemical bulk properties, they do not possess suitable surface properties for biological, pharmacological or medical applications. Organophosphorus acids (phosphoric, phosphonic, and phosphinic) as well as nitrocatechol and derivatives thereof (salts, esters) are highly promising coupling molecules that allow the anchoring of organic groups to inorganic solids, rendering metal oxide surface properties to specific biocompatibility. Colloidal nanocrystals (NCs) have been considered for a multitude of biomedical applications such as bioimaging, drug delivery, photothermal therapy, and radiotherapy enhancement. These NCs are typically hybrid objects consisting of an inorganic core capped with organic ligands. In the case of biomedical applications, controlling the NC surface chemistry is the key as it will play a role in particle agglomeration, cellular uptake, protein adsorption or repelling, cytotoxicity, circulation time, and targeted approaches.
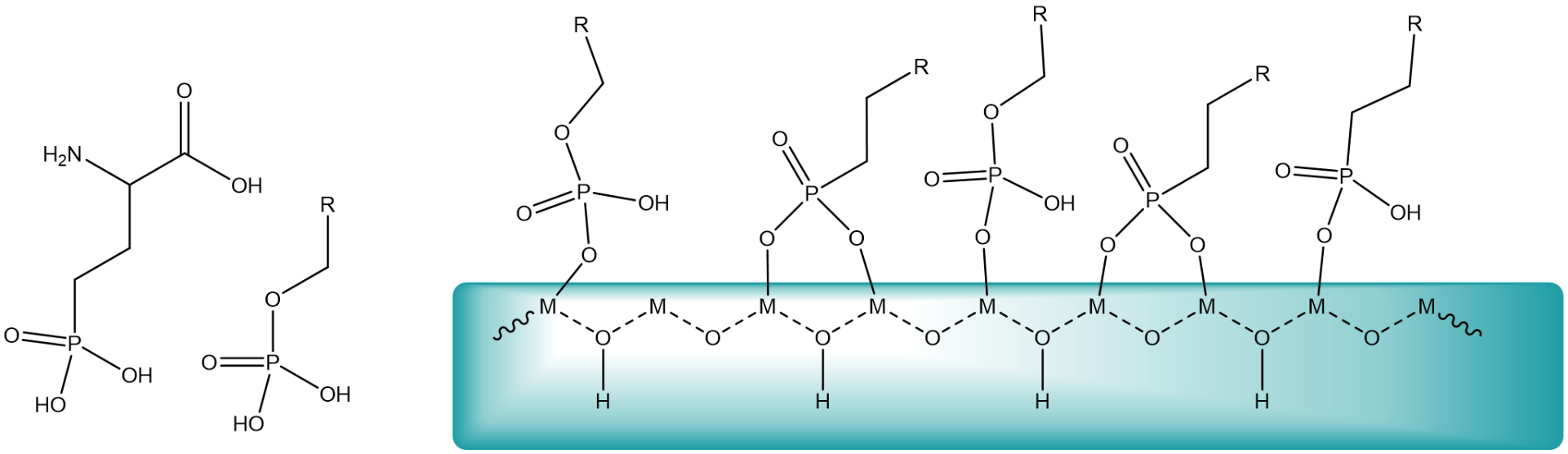
Metal oxides undergo surface complex formation with organic phosphate compounds. The chelate effect of phosphates or phosphonates is the driving force for the binding to the surface. Acids will dissociate as oxygen will be protonated and the metal cation will be coordinated by carboxylate, phosph(on)ate or another anion.
Metal oxide NCs have been particularly successful in nanomedicine and have been reported with compound such as Al2O3, Fe2O3, HfO2, SiO2, SnO2, TiO2, V2O5, or ZrO2. These particles are often first synthesized in nonpolar solvents and stabilized by surfactants (usually with a carboxylate or phosphonate headgroup and an aliphatic tail). Carboxylic acids from fatty acids, for example, dissociate on surface, with carboxylates binding to surface metal sites and the protons to surface oxygen atoms.
The binding affinity of the three most popular ligands for metal oxide nanocrystal surface functionalization are carboxylate, phosph(on)ates, and nitrocatechols. It has been reported that carboxylic acids are the weakest ligands in water - weaker than phosphonic acids or catechols. However, literature is inconclusive as to whether phosphonic acids or catechols are the best ligand as pH influences the ligand solubility through deprotonation of the binding group.
a) Carboxylates bind rather weakly in both nonpolar and polar environments and can be easily displaced by competitive ligands.
b) Phosph(on)ates are strongly bound ligands in alcohols, but desorb in aqueous environment, depending on the pH. They are mostly suited to stabilize nanocrystals in acidic environments.
c) Nitrocatechol derivatives provide a tightly bound ligand shell and excellent stability at physiological and basic pH. They are superior at stabilizing metal oxide nanocrystals in phosphate-buffered saline solutions and cannot be used in acidic environments.
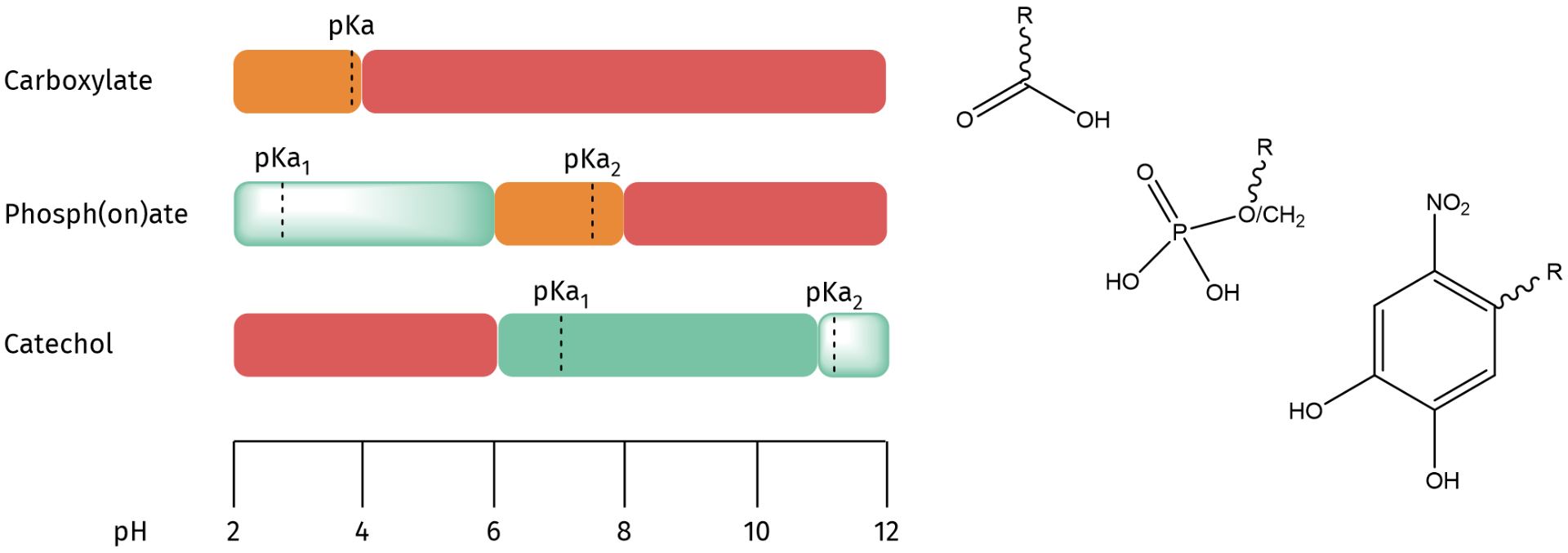
Metal oxide nanocrystals can undergo surface complex formation with different ligands, e.g. carboxylates, phosphates/phosphonates or nitrocatechols. The color code indicates the stability of the nanocrystal-ligand formation at various pH (green = excellent stability; shaded green = high fraction of bound ligands but not perfect; orange = low bound ligand fraction; red = do not use!).
For any specific application, please consult vastly available literature.
→ Interested in linker technologies? Download our brochure Linkerology®
→ For more details on conjugation technologies, click here!
References:
Mapping out the Aqueous Surface Chemistry of Metal Oxide Nanocrystals: Carboxylate, Phosphonate, and Catecholate Ligands; L. Deblock, E. Goossens, R. Pokratath, K. De Buysser, J. De Roo; JACS Au 2022; 2: 711-722. https://doi.org/10.1021/jacsau.1c00565
Adsorption of phosphate on iron oxide doped halloysite nanotubes; D. A. Almasri, N. B. Saleh, M. A. Atieh, G. McKay, S. Ahzi; Sci Rep 2019; 9: 3232. https://doi.org/10.1038/s41598-019-39035-2
Bioconjugate Techniques (Third Edition); G. T. Hermanson; G. T. Hermanson 2013: 188-190. https://doi.org/10.1016/C2009-0-64240-9
Recent advances in separation and detection methods for thiol compounds in biological samples; T. Toyo'oka; J Chromatogr B Analyt Technol Biomed Life Sci 2009; 877: 3318-30. https://doi.org/10.1016/j.jchromb.2009.03.034
Polyethylene glycol-based bidentate ligands to enhance quantum dot and gold nanoparticle stability in biological media; B. C. Mei, K. Susumu, I. L. Medintz, H. Mattoussi; Nat Protoc 2009; 4: 412-23. https://doi.org/10.1038/nprot.2008.243
Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice; G. Zhang, Z. Yang, W. Lu, R. Zhang, Q. Huang, M. Tian, L. Li, D. Liang, C. Li; Biomaterials 2009; 30: 1928-36. https://doi.org/10.1016/j.biomaterials.2008.12.038
Modular poly(ethylene glycol) ligands for biocompatible semiconductor and gold nanocrystals with extended pH and ionic stability; B. C. Mei, K. Susumu, I. L. Medintz, J. B. Delehanty, T. J. Mountziaris, H. Mattoussi; Journal of Materials Chemistry 2008; 18: 4949-4958. https://doi.org/10.1039/b810488c
Surface Modification and Functionalization of Metal and Metal Oxide Nanoparticles by Organic Ligands; M.-A. Neouze, U. Schubert; Monatshefte für Chemie - Chemical Monthly 2008; 139: 183-195. https://doi.org/10.1007/s00706-007-0775-2
Toward reliable gold nanoparticle patterning on self-assembled DNA nanoscaffold; J. Sharma, R. Chhabra, C. S. Andersen, K. V. Gothelf, H. Yan, Y. Liu; J Am Chem Soc 2008; 130: 7820-1. https://doi.org/10.1021/ja802853r
Oriented immobilization of antibodies with GST-fused multiple Fc-specific B-domains on a gold surface; T. H. Ha, S. O. Jung, J. M. Lee, K. Y. Lee, Y. Lee, J. S. Park, B. H. Chung; Anal Chem 2007; 79: 546-56. https://doi.org/10.1021/ac061639+
Simultaneous determination of alpha-lipoic acid and its reduced form by high-performance liquid chromatography with fluorescence detection; S. Satoh, T. Toyo'oka, T. Fukushima, S. Inagaki; J Chromatogr B Analyt Technol Biomed Life Sci 2007; 854: 109-15. https://doi.org/10.1016/j.jchromb.2007.04.003
Design of biotin-functionalized luminescent quantum dots; K. Susumu, H. T. Uyeda, I. L. Medintz, H. Mattoussi; J Biomed Biotechnol 2007; 2007: 90651. https://doi.org/10.1155/2007/90651
Enhanced oligonucleotide-nanoparticle conjugate stability using thioctic acid modified oligonucleotides; J. A. Dougan, C. Karlsson, W. E. Smith, D. Graham; Nucleic Acids Res 2007; 35: 3668-75. https://doi.org/10.1093/nar/gkm237
Biosensing with Luminescent Semiconductor Quantum Dots; K. Sapsford, T. Pons, I. Medintz, H. Mattoussi; Sensors 2006; 6: 925-953. ISSN 1424-8220
Self-assembled organic monolayers: model systems for studying adsorption of proteins at surfaces; K. L. Prime, G. M. Whitesides; Science 1991; 252: 1164-7. https://doi.org/10.1126/science.252.5009.1164