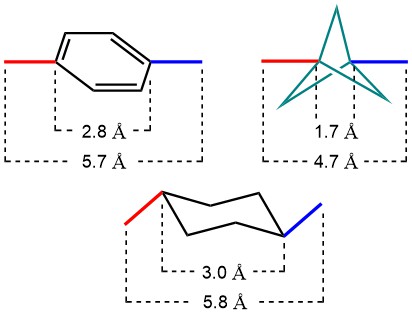
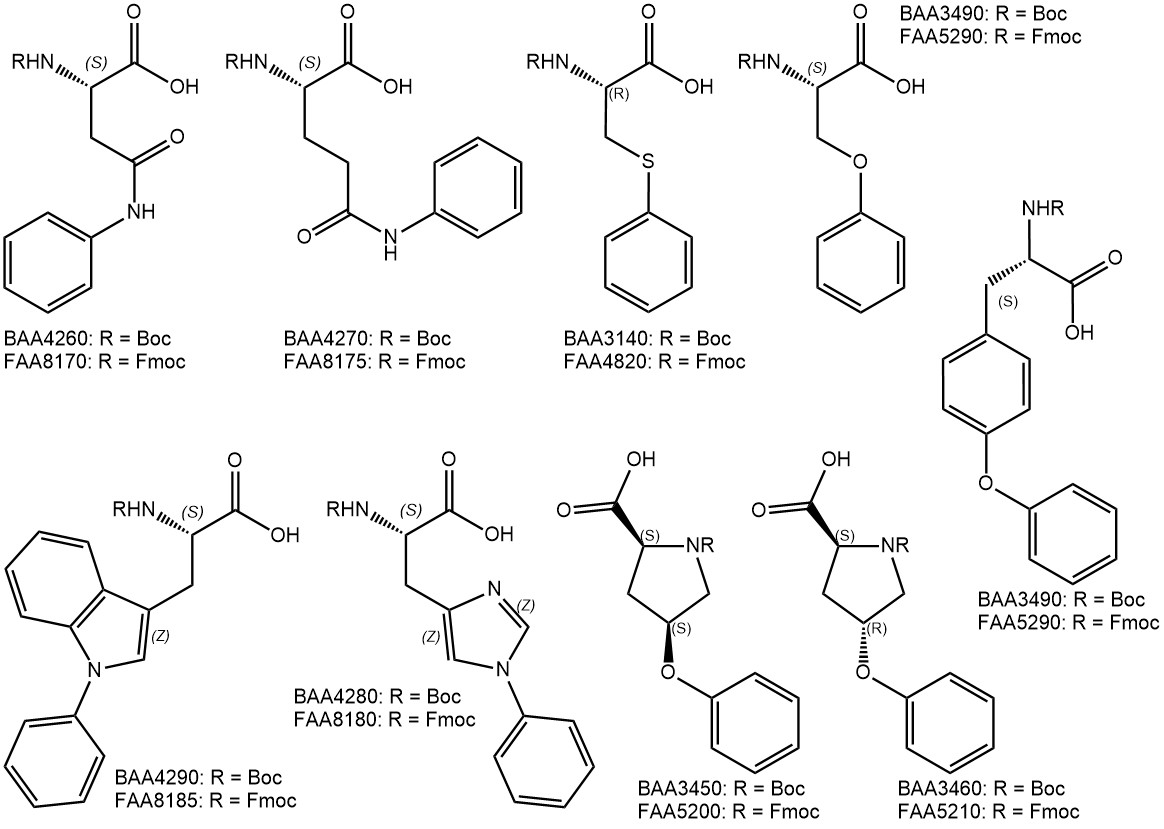
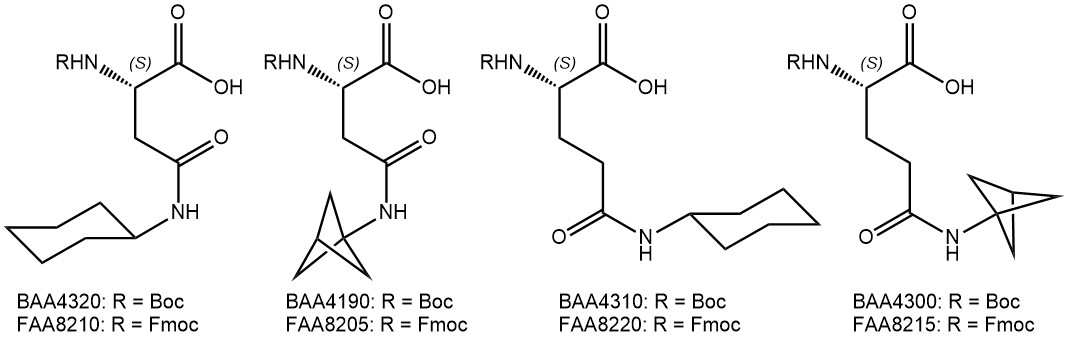
Sidechain-derivatized amino acids have shown great potential in enhancing select properties of peptide lead compounds. Find the most recent additions to our portfolio of amino acids bearing additional aryl and alkyl groups in their side chains.
Adding a phenyl group to the side chain of any amino acid in a given peptide sequence may lead to improved binding properties of the peptide. One example of such a case is the antiviral drug Nelfinavir (Viracept®) which inhibits HIV-1 and HIV-2 proteases. A key structural element of Nelfinavir for optimal binding to HIV protease is an S-phenyl cysteine moiety as a substituent in position P1 (G. Klebe; Pharmazie in unserer Zeit 2001). Interestingly, Nelfinavir has also been shown to strongly inhibit the replication of SARS coronavirus (SARS-CoV) and is currently being tested as a potential treatment for COVID-19 (Yamamoto et al.; Biochem Biophys Res Commun 2004).
In case a lower aromatic ring count is desirable, the phenyl group can be replaced by the bioisostere bicyclo[1.1.1]pentane (BCP) motif. Accordingly derivatized amino acids are now also available from Iris Biotech.
Cyclohexyl groups were introduced as sidechain protecting groups by the Merrifield lab in the early days of peptide chemistry, but soon went out of fashion due to the harsh deprotection conditions required (HF). Similar to phenyl groups, however, cyclohexyl moieties may also be employed to optimize binding of promising lead structures, e.g. leading to much lower IC50 values of a peptide inhibitor against Hepatitic C Virus NS3/4A protease (Nizi et al.; Bioorg Med Chem Lett 2004).
→In case you don’t find your desired derivative in our portfolio, send your inquiry to our Custom Synthesis Service!
References:
- Drug Design Inspired by Nature: Crystallographic Detection of an Auto-Tailored Protease Inhibitor Template; F. M. Gall, D. Hohl, D. Frasson, T. Wermelinger, P. R. E. Mittl, M. Sievers and R. Riedl; Angewandte Chemie (International ed. in English) 2019; 58: 4051-4055. DOI: 10.1002/anie.201812348.
- The Outcomes of Decorated Prolines in the Discovery of Antimicrobial Peptides from Temporin-L; E. Buommino, A. Carotenuto, I. Antignano, R. Bellavita, B. Casciaro, M. R. Loffredo, F. Merlino, E. Novellino, M. L. Mangoni, F. P. Nocera, D. Brancaccio, P. Punzi, D. Roversi, R. Ingenito, E. Bianchi and P. Grieco; ChemMedChem 2019; 14: 1283-1290. DOI: 10.1002/cmdc.201900221.
- HIV protease inhibitors block streptolysin S production; T. Maxson, C. D. Deane, E. M. Molloy, C. L. Cox, A. L. Markley, S. W. Lee and D. A. Mitchell; ACS Chem Biol 2015; 10: 1217-26. DOI: 10.1021/cb500843r.
- Rational design and synthesis of potent and long-lasting glutamic acid-based dipeptidyl peptidase IV inhibitors; T. Y. Tsai, T. Hsu, C. T. Chen, J. H. Cheng, M. C. Chiou, C. H. Huang, Y. J. Tseng, T. K. Yeh, C. Y. Huang, K. C. Yeh, Y. W. Huang, S. H. Wu, M. H. Wang, X. Chen, Y. S. Chao and W. T. Jiaang; Bioorg Med Chem Lett 2009; 19: 1908-12. DOI: 10.1016/j.bmcl.2009.02.061.
- HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus; N. Yamamoto, R. Yang, Y. Yoshinaka, S. Amari, T. Nakano, J. Cinatl, H. Rabenau, H. W. Doerr, G. Hunsmann, A. Otaka, H. Tamamura, N. Fujii and N. Yamamoto; Biochem Biophys Res Commun 2004; 318: 719-25. DOI: 10.1016/j.bbrc.2004.04.083.
- Discovery of potent antagonists of the antiapoptotic protein XIAP for the treatment of cancer; T. K. Oost, C. Sun, R. C. Armstrong, A. S. Al-Assaad, S. F. Betz, T. L. Deckwerth, H. Ding, S. W. Elmore, R. P. Meadows, E. T. Olejniczak, A. Oleksijew, T. Oltersdorf, S. H. Rosenberg, A. R. Shoemaker, K. J. Tomaselli, H. Zou and S. W. Fesik; J Med Chem 2004; 47: 4417-26. DOI: 10.1021/jm040037k.
- Capped dipeptide phenethylamide inhibitors of the HCV NS3 protease; E. Nizi, U. Koch, J. M. Ontoria, A. Marchetti, F. Narjes, S. Malancona, V. G. Matassa and C. Gardelli; Bioorg Med Chem Lett 2004; 14: 2151-4. DOI: 10.1016/j.bmcl.2004.02.032.
- Biphenyls as potent vitronectin receptor antagonists. Part 2: biphenylalanine ureas; K. Urbahns, M. Härter, A. Vaupel, M. Albers, D. Schmidt, U. Brüggemeier, B. Stelte-Ludwig, C. Gerdes and H. Tsujishita; Bioorganic & Medicinal Chemistry Letters 2003; 13: 1071-1074. DOI: 10.1016/s0960-894x(03)00023-4.
- Novel, potent phenethylamide inhibitors of the hepatitis C virus (HCV) NS3 protease: probing the role of P2 aryloxyprolines with hybrid structures; F. Orvieto, U. Koch, V. G. Matassa and E. Muraglia; Bioorg Med Chem Lett 2003; 13: 2745-8. DOI: 10.1016/s0960-894x(03)00536-5.
- Wirkstoffdesign bei der Entwicklung substratähnlicher HIV-Protease-Hemmstoffe: Molecular Modelling im Kampf gegen AIDS; G. Klebe; Pharmazie in unserer Zeit 2001; 30: 194-201. DOI: 10.1002/1615-1003(200105)30:33.0.Co;2-9.
- Solid phase synthesis of the protected 43-55 tridecapeptide of the heavy chain of myeloma immunoglobin M603, employing cyclohexyl ester protection for glutamic acid; R. D. Dimarchi, J. P. Tam and R. B. Merrifield; International journal of peptide and protein research 1982; 19: 270-9. DOI: 10.1111/j.1399-3011.1982.tb03038.x.
- Cyclohexyl ester as a new protecting group for aspartyl peptides to minimize aspartimide formation in acidic and basic treatments; J. P. Tam, T.-W. Wong, M. W. Riemen, F.-S. Tjoeng and R. B. Merrifield; Tetrahedron Letters 1979; 20: 4033-4036. DOI: 10.1016/s0040-4039(01)86496-0.