Potential alternatives to PEGs, polypeptoids in general and polysarcosine (PSR) in particular stand out in terms of safety, synthetic control and versatility.
2.4 Polysarcosine – a True Alternative to PEG
The PEGylation technology has transformed the fields of bioconjugation, drug delivery and nanomedicine tremendously. PEGylation of surfaces, drugs and biologics has become a multi-billion euro business. However, a heavily crowded patent landscape, reports of patients failing treatment due to anti-PEG antibodies and concerns over the long-term safety of PEG have triggered intensive research efforts to find suitable alternative technologies. Among those potential alternatives, polypeptoids in general and polysarcosine (PSR) in particular stand out in terms of safety, synthetic control and versatility.
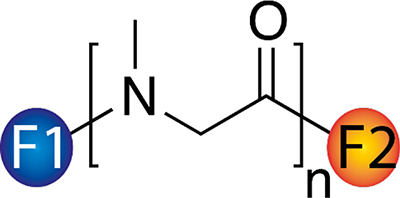
Monofunctional, homo- and heterobifunctional PSR with a wide variety of functional groups F1 and F2 are offered. Degrees of polymerization n may range from below 10 to above 1.000. Thus, molar masses of approx. 1 kg/mol to 100 kg/mol are possible. In brief, PSR is characterized by the following properties:
- Biobased, degradable and non-immunogenic. ff Low protein adsorption.
- Excellent water solubility and solubility in organic solvents.
- Highly defined polymers with narrow poissondistribution.
- Mono-, homo- and heterobifunctional; customdesigned functionalities upon request.
- Excellent shelf-life, reproducibility and analytical purity.
Polysarcosine – The biobased macromolecular tool with excellent dispersity and designability
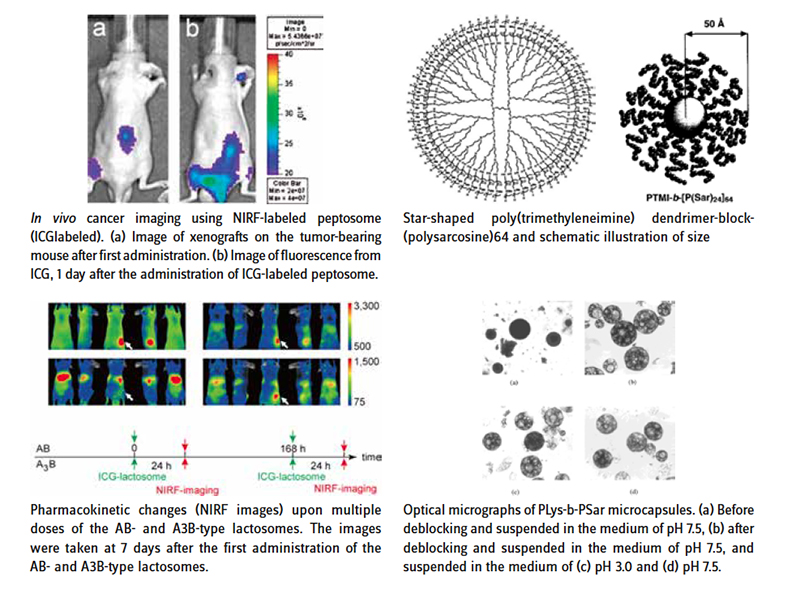
Polysarcosine (PSR) – originating from the natural, non-toxic amino acid sarcosine (N-Methylglycine) – is the simplest polypeptoid and a newly rediscovered biocompatible and degradable polymer. Polysarcosine has been employed in a number of drug delivery systems, including dendrimers [1,2], polymer micelles [3,4], polyplexes [5], protein conjugates [6,7] and micro-[8-11] and nanoparticles [12-18], polymersomes [19] and nanotubes [20,21]. However, widespread use of PSR has been hampered by lack of commercially-available functional PSR in good quality. This fact is now changed by us! The use of PSR with functional head- and tail-groups for bioconjugation is comparable to the well-known PEGylation technology. A wide range of functional terminal groups can be realized. However, in contrast to PEG, PSR is intrinsically heterobifunctional (-COOH, -NH2). Therefore, the scope of the heterofunctional building-block design is extensive. As an early adopter of the PSR technology, you will have a competitive advantage over users of the ever-present PEGylation. Functional polysarcosine offers a great opportunity to create innovation and opportunities in many different fields of applications. Don´t allow your creativity to be limited! Furthermore, PSR is a hydrophilic polymer [22] that shows excellent non-fouling properties leading to proteinrepellent surfaces [23,24] and long-circulating polymers or polymer nanoparticles [17,18]. Moreover, it is degradable under physiologically relevant conditions [25], exhibits low immunogenicity [26,27] and low toxicity. PSR is accessible via nucleophilic living condensative ring-opening polymerization (NuLCROP) of sarcosine N-carboxyanhydride, and thus is highly defined with very low dispersities (Poisson distribution) and excellent reproducibility [28]. We offer a large variety of attractive chemical functionalities such as amines, azides, alkynes and thiols for bioconjugation to drugs, proteins and surfaces of your choice. We also offer a range of molar masses, favorable for bioconjugation and use as biomaterials. Conveniently, for further modifications, PSR exhibits excellent water solubility and solubility in a wide range of organic solvents [22]. To analyze your bioconjugate conveniently, the polymer is UV-active at 200-220 nm, which allows detection using standard HPLC equipment and UV-Vis spectrometers. In summary, PSR technology is a novel toolkit of nonionic, non-toxic and non-immunogenic hydrophilic and organosoluble polymers with a wide range of functionalities.
References:
- Synthesis of a novel star-shaped dendrimer by radial-growth polymerization of sarcosine N-carboxyanhydride initiated with poly(trimethyleneimine) dendrimer; K. Aoi, T. Hatanaka, K. Tsutsumiuchi, M. Okada and T. Imae; Macromol Rapid Commun 1999; 20: 378-382. doi:10.1002/(sici)1521-3927(19990701)20:7 3.0.co;2-s
- Molecular assembly composed of a dendrimer template and block polypeptides through stereocomplex formation; H. Matsui, M. Ueda, A. Makino and S. Kimura; Chem Commun 2012; 48: 6181-6183. doi:10.1039/c2cc30926b
- Factors Influencing in Vivo Disposition of Polymeric Micelles on Multiple Administrations; E. Hara, M. Ueda, A. Makino, I. Hara, E. Ozeki and S. Kimura; ACS Med Chem Lett. 2014; 5: 873-877. doi:10.1021/ml500112u
- Suppressive immune response of poly-(sarcosine) chains in peptidenanosheets in contrast to polymeric micelles; E. Hara, M. Ueda, C. J. Kim, A. Makino, I. Hara, E. Ozeki and S. Kimura; J Pept Sci 2014; 20: 570-577. doi:10.1002/psc.2655
- Introducing PeptoPlexes: Polylysine-block-Polysarcosine Based Polyplexes for Transfection of HEK 293T Cells; P. Heller, A. Birke, D. Huesmann, B. Weber, K. Fischer, A. Reske-Kunz, M. Bros and M. Barz; Macromol Biosci 2014; 14: 1380-1395. doi:10.1002/mabi.201400167
- Suppression of Murine IgE Responses with Amino Acid Polymer/ Allergen Conjugates; D. C. Henderson, A. W. Wheeler and D. M. Moran; Int Arch Allergy Appl Immunol 1987; 82: 208-211. doi:10.1159/000234188
- Suppression of Murine IgE Responses with Amino Acid Polymer/ Allergen Conjugates; N. Whittall, D. M. Moran, A. W. Wheeler and G. P. Cottam; Int Arch Allergy Appl Immunol 1985; 76: 354-360. doi:10.1159/000233721
- pH-responsive release from polypeptide microcapsules; T. Kidchob, S. Kimura and Y. Imanishi; J Appl Polym Sci 1997; 63: 453-458. doi:10.1002/ (sici)1097-4628(19970124)63:43.0.co;2-q
- Thermoresponsive release from poly(Glu(OMe))-block-poly(Sar) microcapsules with surface-grafting of poly(N-isopropylacrylamide); T. Kidchob, S. Kimura and Y. Imanishi; J Control Release 1998; 50: 205-214. doi:10.1016/S0168-3659(97)00135-1
- Amphiphilic poly(Ala)-b-poly(Sar) microspheres loaded with hydrophobic drug; T. Kidchob, S. Kimura and Y. Imanishi; J Control Release 1998; 51: 241-248. doi:10.1016/S0168-3659(97)00176-4
- Controlled release from amphiphilic polymer aggregates; S. Kimura, T. Kidchob and Y. Imanishi; Polym Advan Technol 2001; 12: 85-95. doi:10.1002/1099-1581(200101/02)12:1/23.0.co;2-8
- Polypeptoid-block-polypeptide Copolymers: Synthesis, Characterization, and Application of Amphiphilic Block Copolypept(o) ides in Drug Formulations and Miniemulsion Techniques; A. Birke, D. Huesmann, A. Kelsch, M. Weilbächer, J. Xie, M. Bros, T. Bopp, C. Becker, K. Landfester and M. Barz; Biomacromolecules 2014; 15: 548- 557. doi:10.1021/bm401542z
- Radiosynthesis and initial evaluation of 18F labeled nanocarrier composed of poly(L-lactic acid)-block-poly(sarcosine) amphiphilic polydepsipeptide; F. Yamamoto, R. Yamahara, A. Makino, K. Kurihara, H. Tsukada, E. Hara, I. Hara, S. Kizaka-Kondoh, Y. Ohkubo, E. Ozeki and S. Kimura; Nucl Med Biol 40: 387-394. doi:10.1016/j. nucmedbio.2012.12.008
- Pharmacokinetic change of nanoparticulate formulation “Lactosome” on multiple administrations; E. Hara, A. Makino, K. Kurihara, F. Yamamoto, E. Ozeki and S. Kimura; Int Immunopharmaco 2012; 14: 261-266. doi:10.1016/j.intimp.2012.07.011
- Control of in vivo blood clearance time of polymeric micelle by stereochemistry of amphiphilic polydepsipeptides; A. Makino, E. Hara, I. Hara, R. Yamahara, K. Kurihara, E. Ozeki, F. Yamamoto and S. Kimura; J Control Release 2012; 161: 821-825. doi:10.1016/j. jconrel.2012.05.006
- Transformation of peptide nanotubes into a vesiclevia fusion driven by stereo-complex formation; M. Ueda, A. Makino, T. Imai, J. Sugiyama and S. Kimura; Chem Commun 2011; 47: 3204-3206. doi:10.1039/c0cc04209a
- Near-infrared fluorescence tumor imaging using nanocarrier composed of poly(l-lactic acid)-block-poly(sarcosine) amphiphilic polydepsipeptide; A. Makino, S. Kizaka-Kondoh, R. Yamahara, I. Hara, T. Kanzaki, E. Ozeki, M. Hiraoka and S. Kimura; Biomaterials 2009; 30: 5156-5160. doi:10.1016/j.biomaterials.2009.05.046
- Near-Infrared Fluorescent Labeled Peptosome for Application to Cancer Imaging; H. Tanisaka, S. Kizaka-Kondoh, A. Makino, S. Tanaka, M. Hiraoka and S. Kimura; Bioconjug Chem 2008; 19: 109- 117. doi:10.1021/bc7001665
- Temperature-Triggered Fusion of Vesicles Composed of Right-Handed and Left-Handed Amphiphilic Helical Peptides; M. Ueda, A. Makino, T. Imai, J. Sugiyama and S. Kimura; Langmuir 2011; 27: 4300-4304. doi:10.1021/la105140v
- Rational design of peptide nanotubes for varying diameters and lengths; M. Ueda, A. Makino, T. Imai, J. Sugiyama and S. Kimura; J Pept Sci 2011; 17: 94-99. doi:10.1002/psc.1304
- Self-Assemblies of Triskelion A2B-Type Amphiphilic Polypeptide Showing pH-Responsive Morphology Transformation; A. Uesaka, M. Ueda, A. Makino, T. Imai, J. Sugiyama and S. Kimura; Langmuir 2012; 28: 6006-6012. doi:10.1021/la3004867
- Polypeptoids from N-Substituted Glycine N-Carboxyanhydrides: Hydrophilic, Hydrophobic, and Amphiphilic Polymers with Poisson Distribution; C. Fetsch, A. Grossmann, L. Holz, J. F. Nawroth and R. Luxenhofer; Macromolecules 2011; 44: 6746-6758. doi:10.1021/ma201015y
- An Experimental–Theoretical Analysis of Protein Adsorption on Peptidomimetic Polymer Brushes; K. H. A. Lau, C. Ren, S. H. Park, I. Szleifer and P. B. Messersmith; Langmuir 2012; 28: 2288-2298. doi:10.1021/la203905g
- Surface-Grafted Polysarcosine as a Peptoid Antifouling Polymer Brush; K. H. A. Lau, C. Ren, T. S. Sileika, S. H. Park, I. Szleifer and P. B. Messersmith; Langmuir 2012; 28: 16099-16107. doi:10.1021/la302131n
- On the biodegradability of polyethylene glycol, polypeptoids and poly(2-oxazoline)s; J. Ulbricht, R. Jordan and R. Luxenhofer; Biomaterials 2014; 35: 4848-4861. doi:10.1016/j. biomaterials.2014.02.029
- Antigenicity of Polypeptides (Poly Alpha Amino Acids); P. H. Maurer, D. Subrahmanyam, E. Katchalski and E. R. Blout; J Immunol 1959; 83: 193-197.
- Immunological studies with synthetic polypeptides; M. Sela; Adv Immunol 1966; 5: 29-129.
- Polypeptoids: A perfect match for molecular definition and macromolecular engineering?; R. Luxenhofer, C. Fetsch and A. Grossmann; J. Polym. Sci.: Part A: Polym. Chem. 2013; 51: 2731-2752. doi:10.1002/pola.26687