Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 09.11.2011
Product of the month: April
Cleland's reagent, also known as dithiothreitol or DTT is a water soluble protective reagent for sulfhydryl groups.
It reduces disulfide linkages to free sulfhydryl groups in proteins and enzymes.
It is a component of buffers used in protocols for the isolation and purification of proteins.

|
Synonyms: |
DL-Dithiothreitol, (2S,3S;2R,3R)-threo-1,4-Dimercapto-2,3-butandiol |
| Code | RL-1020 | |
| 25g: | EUR 125 | US$ 200 |
| 100g: | EUR 300 | US$ 480 |
| 250g | EUR 450 | US$ 720 |
Prices are in EUR/USD, net, exw Germany, shipping not include
Our production process does not involve carcinogenic intermediates. It is therefore a safe process, taking care of the integrity of environment and of the health of all personal involved in production and handling.
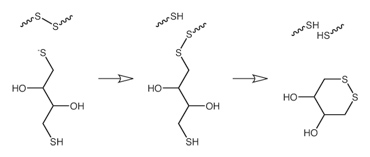
DTT is very strong reducing agent, due to the property to form a six-membered ring with an internal disulfide bond in oxidised form.
The redox potential is -0.33 V at pH 7. The pKa values of the thiol groups are 9.2 and 10.1 respectively.
The reduction of a typical disulfide bond proceeds by two sequential thiol-disulfide exchange reactions as illustrated above. The reducing power of DTT is limited to pH values above 7, since the negatively charged thiolate form only is the reactive agent in opening disulfide bonds in proteins.
A common use of DTT is as a deprotecting thiolated DNA. The terminal sulfurs of thiolated DNA have a tendency to oxidize and form dimers in solution, especially in the presence of oxygen. Dimerization significantly lowers the efficiency of subsequent coupling reactions such as DNA immobilization on gold surfaces in biosensors. Normally DTT is mixed with a DNA solution and allowed to react, and then is removed by filtration (solid catalyst) or by chromatography (liquid form). The DTT removal procedure is also commonly called "desalting."
DTT is frequently used to reduce the disulfide bonds of proteins and, in order to prevent intramolecular (Cyclization) and intermolecular (Oligomerisation, Polymerisation) disulfide bonds from cysteine residues of proteins. However, DTT cannot reduce solvent-inaccessible disulfide bonds, so reduction of disulfide bonds is sometimes carried out under denaturing conditions (e.g., at high temperatures, or in the presence of a strong denaturating agnets such as 6 M guanidinium hydrochloride, 8 M urea, or 1% sodium dodecylsulfate). Conversely, the solvent exposure of different disulfide bonds can be assayed by their rate of reduction in the presence of DTT.
DTT can also be used as an oxidizing agent. Its inherent advantage is that effectively no mixed-disulfide species will be formed, which can occur with other agents such as glutathione.
|
Literature: Dithiothreitol, A New Protective Reagent for SH Groups; Cleland, W.W.; Biochemistry; April 1964; 3: 480–2. doi:10.1021/bi00892a002. Cleavage of disulfide bonds in proteins; Ruegg, UT; Rudinger, J.; Methods Enzymol 1977; 47: 111. From Production of Peptides in Milligram Amounts for Research to Multi-Tons Quantities for Drugs of the Future; Thomas Bruckdorfer, Oleg Marder and Fernando Albericio; Current Pharmaceutical Biotechnology 2004; 5: 29-43. |