Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 26.01.2021
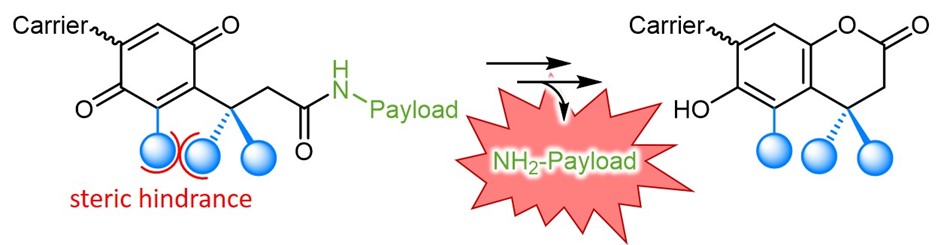
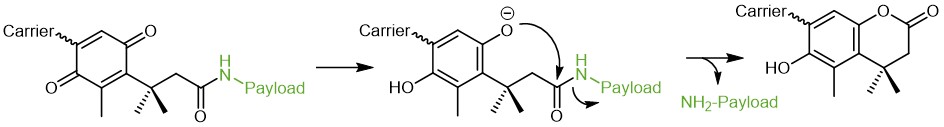
As scientists aim to control the reactivity and activity of their biomolecules in a spatial and/or temporal manner, diverse tools for selected, trigger-induced drug delivery have been investigated. These allow to release the biological activity only in the targeted environment and, thereby minimizing systemic adverse effects and improving the overall pharmacological performance. One strategy uses the trimethyl lock (TML) – an ortho-hydroxy dihydro cinnamic acid derivative. Its name arises from the three sterically interfering methyl groups, which are placed for improved reactivity and fast drug release upon lactonization and dihydro coumarin formation.
Schematic illustration of the concept of trimethyl locks.
Blocking of the phenolic hydroxyl group, e.g. by acetylation, or masking of the reactivity as benzoquinone derivate prevents lactonization. Depending on the functional group, TMLs can be designed to respond to a distinct trigger. As an example, phenyl ester protected TMLs are esterase-responsive, phosphate esters respond to phosphatases.
A recent publication (J. Med. Chem. 2020; 63(19): 11034-11044) describes the discovery of an alkaline phosphatase-responsive trimethyl-lock-based solubilizing prodrug of the HCV NS5A inhibitor pibrentasvir (PIB). Following dephosphorylation, rapid lactonization of the ortho-hydroxyphenyl dimehtlypropionate results in efficient drug release.
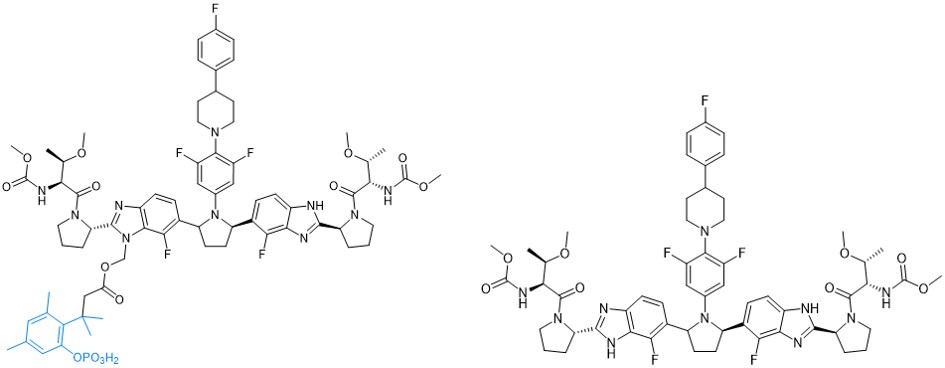
Chemical structure of an alkaline-phosphatase sensitive trimethyl-lock prodrug of the HCV NS5A inhibitor pibrentasvir (left) and pibrentasvir (right) (J. Med. Chem. 2020; 63(19): 11034-11044).
Nitro-reductase sensitive systems rely on the reduction of the para-nitro benzyl moiety to the corresponding self-immolative para-amino benzyl unit releasing an unmasked TML via 1,4-elimination, thus undergoing rapid lactonization and payload release. Nitro-reductases are used as biomarker as those enzymes are overexpressed particularly in hypoxic tissue, such as cancer cells.
Besides enzymatic activation and quinone masking, the use of light to initiate reactions can lead to very clean and precisely controlled processes. The combination of photochemical triggers with the TML principle is therefore a powerful release system. Iris Biotech offers the Photo-Trimethyl Lock RL-2970.
In addition to classical trimethyl lock derivatives, Iris Biotech also offers the fourmethyl-lock RL-2950 and even the fivemethyl-lock RL-2940. Find more TML derivatives within the related products section at the end of this page.
Interested in further information on linker technologies? -> Please feel free to download our new brochure on Linkerology!
References:
Prodrug Strategies to Improve the Solubility of the HCV NS5A Inhibitor Pibrentasvir (ABT-530); J. T. Randolph, E. A. Voight, S. N. Greszler, B. E. Uno, J. N. Newton, K. M. Gleason, D. Stolarik, C. Van Handel, D. A. J. Bow, D. A. DeGoey; J. Med. Chem. 2020; 63(19): 11034-11044. https://doi.org/10.1021/acs.jmedchem.0c00956.
Mechanistic Studies of the Photoinduced Quinone Trimethyl Lock Decaging Process; C. J. Regan, D. P. Walton, O. S. Shafaat, D. A. Dougherty; J. Am. Chem. Soc. 2017; 139(13): 4729-4736. https://doi.org/10.1021/jacs.6b12007.
Trimethyl lock: A trigger for molecular release in chemistry, biology, and pharmacology; M. N. Levine, R. T. Raines; Chem. Sci. 2012; 3: 2412-2420. https://doi.org/c2sc20536j.
Photo-triggered fluorescent labelling of recombinant proteins in live cells; D. Jung, K. Sato, K. Min, A. Shigenaga, J. Jung, A. Otaka, Y. Known; Chem. Commun. 2015; 51: 9670-3. https://doi.org/10.1039/c5cc01067e.
Detection of DT-diaphorase Enzyme with a ParaCEST MRI Contrast Agent; I. Daryaei, K. M. Jones, M. D. Pagel; Chem. Eur. J. 2017; 23: 6514-6517. https://doi.org/10.1002/chem.201700721.
Syntheses and kinetic studies of cyclisation-based self-immolative spacers; S. Huvelle, A. Alouane, T. Le Saux, L. Jullien, F. Schmidt; Org. Biomol. Chem. 2017; 15: 3435-3443. https://doi.org/10.1039/c7ob00121e.
Invention of stimulus-responsive peptide-bond-cleaving residue (Spr) and its application to chemical biology tools; A. Shigenaga, J. Yamamoto, T. Kohiki, T. Inokuma, A. Otaka; J. Pept. Sci. 2017; 23: 505-513. https://doi.org/10.1002/psc.2961.
Trimethyl Lock: A Multifunctional Molecular Tool for Drug Delivery, Cellular Imaging, and Stimuli-Responsive Materials; O. A. Okoh, P. Klahn; ChemBioChem 2018; 19: 1668-1694. https://doi.org/10.1002/cbic.201800269.