Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 09/02/2017
Trypsin (EC 3.4.21.4), an endopeptidase and natural protease found in the digestive system, cleaves peptide chains and proteins predominantly at the carboxyl side of the amino acids Lys and Arg (except when followed by Pro). Peptides and proteins are gaining importance as modern biopharmaceuticals, due to their high specifity and efficacy. One drawback, however, is their low stability and resulting unfavourable pharmacokinetics. Proteases such as trypsin, in fact, degrade peptides and proteins rather rapidly decreasing in this way their bioavailability. It has, however, been demonstrated that shorter homologues of arginine built into model peptides will increase their stability accordingly. Similar effects have been observed whenever the guanidine side chain has been modified.
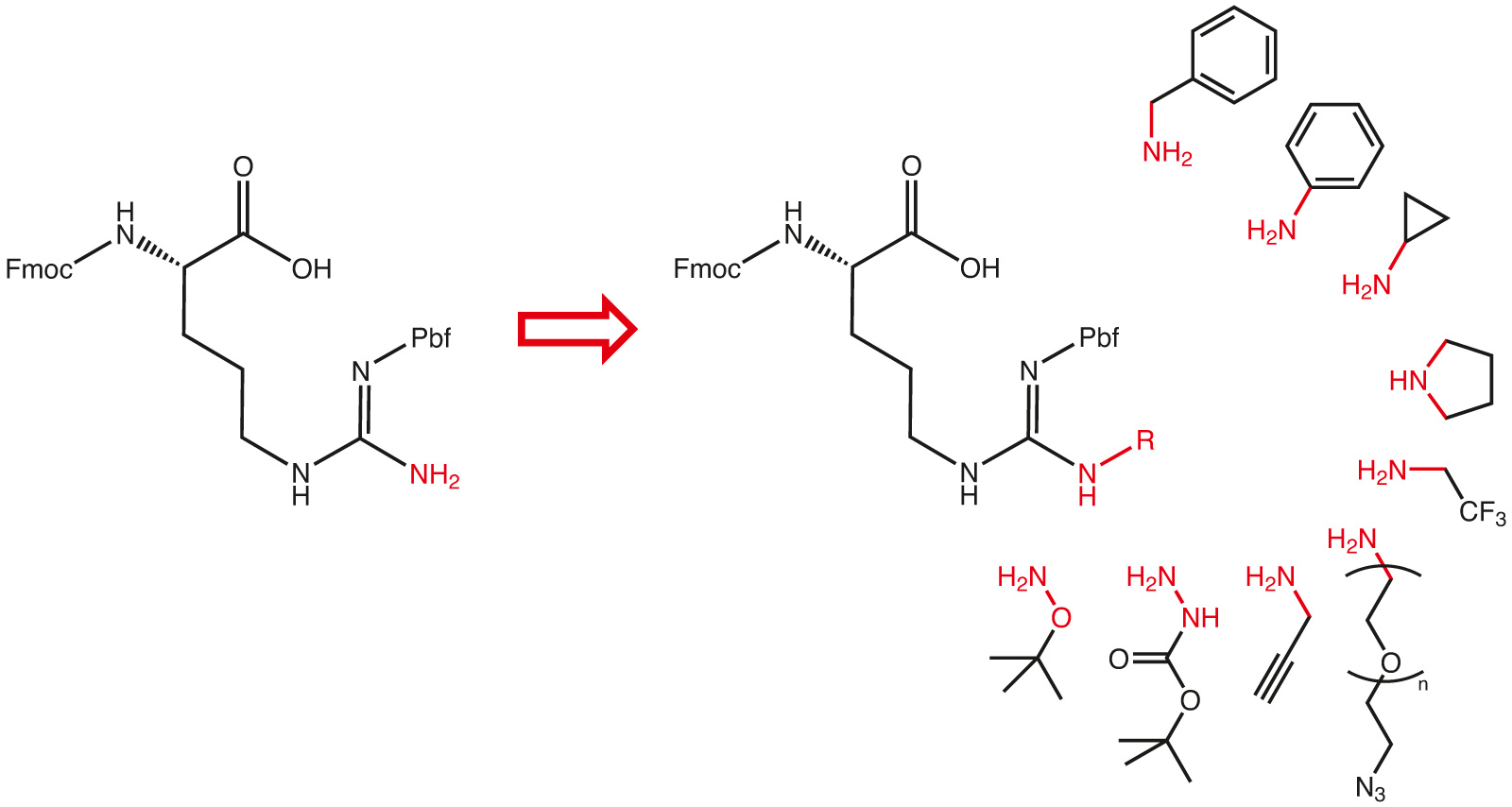
With our proprietary arginine platform we can design arginine derivatives bearing substitutions on the guanidine nitrogen from any commercially available amine. A process is available to provide Fmoc and Pbf protected derivatives, which can instantly be used in any Fmoc/tBu solid phase peptide synthesis protocol.