Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 21/02/2022
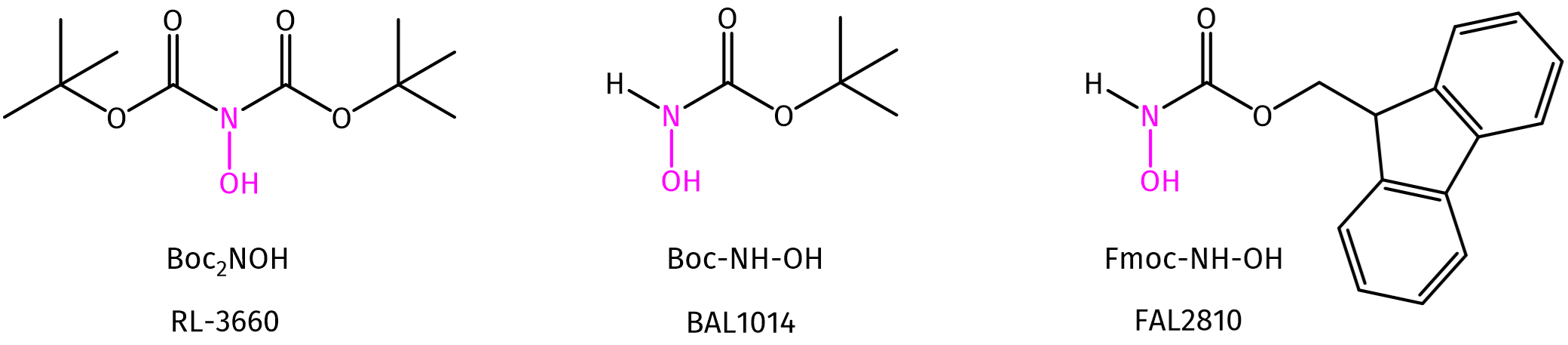
The selective oxidation of primary amines (NH2) to hydroxylamines (HN-OH) is a crucial synthetic transformation as the latter species is highly reactive and oxidatively labile.

Oxidation of a primary amine to its nitro derivative via hydroxylamine and nitroso.
Other routes for the introduction of hydroxylamines include for example the reductive amination of aldehydes which involves the conversion of a carbonyl group to the hydroxylamine via the corresponding N-hydroxyimine.
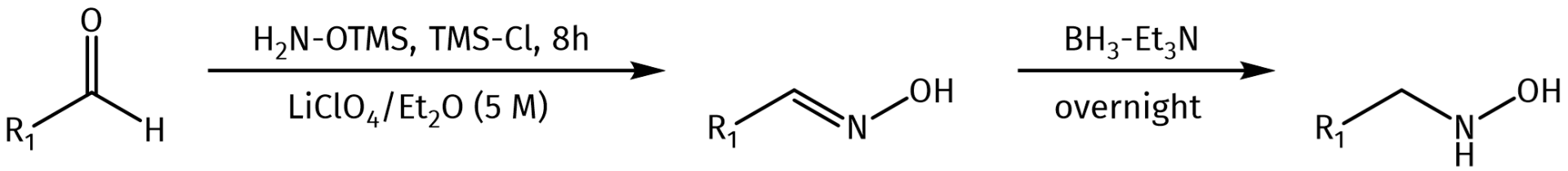
Synthesis of N-Hydroxylamine compounds via reductive amination as published by Albericio et al.
Herein, we present protected (Fmoc/Boc) hydroxylamine building blocks. These reagents can be used for the synthesis of various protected hydroxylamine derivatives. Besides, in literature, the N,N’-Di-tert-butoxycarbonylhydroxylamine [(Boc)2NOH] building block (RL-3660) is reported for the efficient synthesis of alkoxyamine derivatives from alkyl bromides. Amongst other applications, alkoxyamines can be used for cyclization reactions and ligation. Interested in aminooxy-derivatives and their applications? See our blog and discover more!
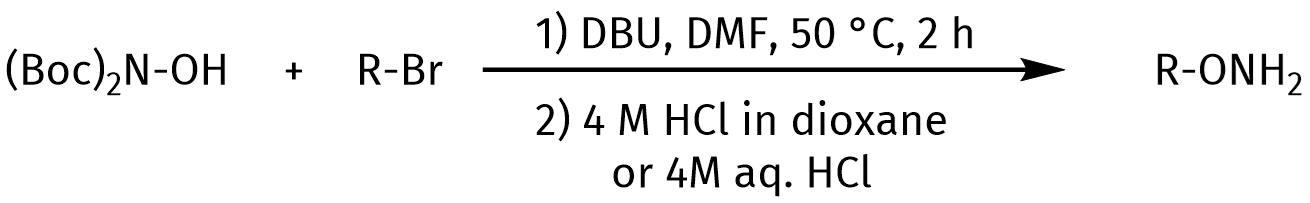
Synthesis of alkoxyamines via reaction of diBoc-hydroxylamine with alkylbromide.
Such hydroxylamines and aminooxy derivatives can be further used for bio-conjugation or other diverse applications. Besides, hydroxylamines can undergo a variety of reactions. Especially the formation of oximes upon reaction with aldehydes/ketones is useful for example in the purification of the last mentioned. If hydroxylamine is added to an aldehyde or ketone in solution, the oxime forms, which generally precipitates from solution and can be reconverted to the respective aldehyde/ketone.
Besides, N-substituted hydroxylamines are reported in literature for their antibacterial activity against both gram-positive and gram-negative bacteria helping to fight multidrug-resistant bacteria, a major global healthcare problem in the 21st century.
References:
Rapid Synthesis of Alkoxyamine Hydrochloride Derivatives from Alkyl Bromide and N,N’-Di-tert-butoxycarbonylhydroxylamine [(Boc)2NOH]; P. S. Jayasekara, K. A. Jacobson; Synth. Commun. 2014; 44: 2344-2347. https://doi.org/10.1080/00397911.2014.895014.
In situ growth of a stoichiometric PEG-like conjugate at a protein’s N-terminus with significantly improved pharmacokinetics; W. Gao, W. Liu, J. A. Mackay, M. R. Zalutsky, E. J. Toone, A. Chilkoti; PNAS 2009; 106(36): 15231-15236. https://doi.org/10.1073/pnas.0904378106.
4-Alkyloxyimino Derivatives of Uridine-5’-trphosphate: Distal Modification of Potent Agonists as a Strategy for Molecular Probes of P2Y2, P2Y4, and P2Y6 Receptors; P. S. Jayasekara, M. O. Barrett, C. B. Ball, K. A. Brown, E. Hammes, R. Balasubramanian, T. K. Harden, K. A. Jacobson; J. Med. Chem. 2014; 57: 3874-3883. https://doi.org/10.1021/jm500367e.
Synthesis of Constrained Tetracyclic Peptides by Consecutive CEPS, CLIPS, and Oxime Ligation; D. E. Streefkerk, M. Schmidt, J. H. Ippel, T. M. Hackeng, T. Nuijens, P. Timmerman, and J. H. van Maarseveen; Org. Lett. 2019; 21(7): 2095-2100. https://doi.org/10.1021/acs.orglett.9b00378.
A Reagent for the Convenient, Solid-Phase Synthesis of N-Terminal Peptide Hydroxylamines for Chemoselective Ligations; T. Fukuzumi, J. W. Bode; J. Am. Chem. Soc. 2009; 131(11): 3864-3865. https://doi.org/10.1021/ja900601c.
Hydroxylamine Derivatives as a New Paradigm in the Search of Antibacterial Agents; L. Miret-Casals, A. Baelo, E. Julián, J. Astola, A. Lobo-Ruiz, F. Albericio, E. Torrents; ACS Omega 2018; 3(12): 17057-17069. https://doi.org/10.1021/acsomega.8b01384.