Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 07/02/2017
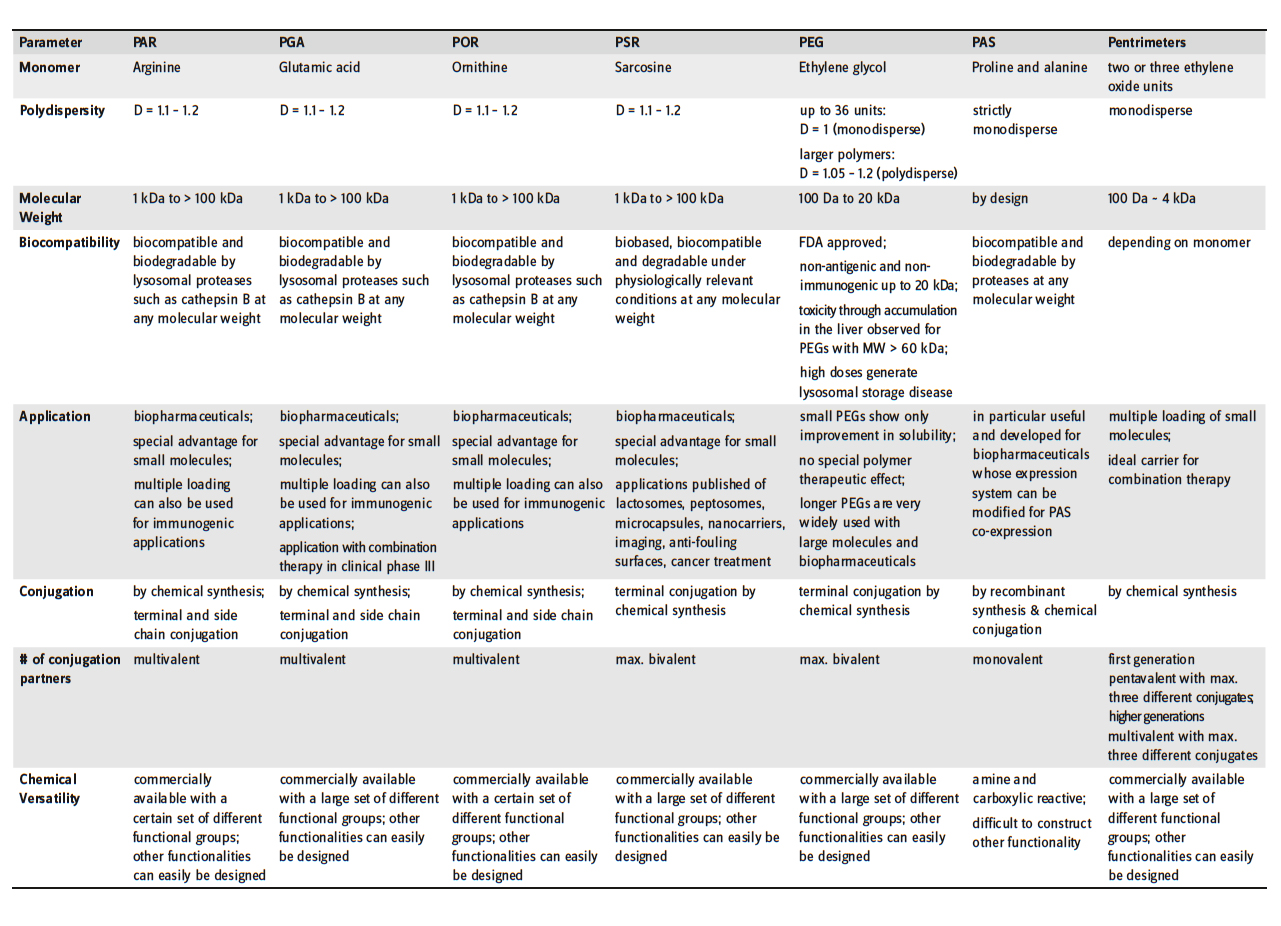
1.2 Polydispersity
The polymers in this context are a polymeric linear structures with n repeating units of monomers. Depending whether the polymer is consisting of one single molecular weight (only one n existing) or of a range of compounds with an average mass and a distribution of n around a mean value, polymers are referred to as “monodisperse” or “polydisperse”. If the polymer is polydisperse it shows a mass spectrum as shown in the figure. In order to quantify the distribution of the molecular weight, the Polydispersity D is defined as the ratio between the weight average molecular weight Mw ° and the number average molecular weight Mn °. The weight average molecular weight does not “count” species just by their number, but takes into account the total weight of each species and is therefore a much more realistic indicator of the gross mechanical property. For a homogeneous sample, where the polymer chains have all the same length, Mw ° is equal to Mn °, the polydispersity D is then equal to 1 and the sample is referred to be monodisperse. Whenever there is a distribution of molecular weights, the weight average Mw ° is always greater than the number average Mn ° and the polydispersity is greater than 1. The polydispersity D of PEGs, PGAs and PSRs typically used in polymer therapeutics is between 1.05 and 1.20. Though, whenever a PEGylated new drug compound needs to be approved by EMEA, FDA and other authorities, it is easier and faster if this compound shows only one signal in the mass spectrum and not a distribution pattern. Therefore the need for high molecular weight but monodisperse compounds is increasing. PAS polymers are the choice in this case. As they are produced through recombinant methods, only one specific molecular weight exists although it is a large molecule.
Reference:
1.3 Alternative Carriers for Polymer Therapeutics
Polymer Therapeutics (PT) can be underlined as the most successful first generation nanomedicines according to the 2014 report on the US Top 10 selling drugs that include the polymeric drug Glatiramer acetate for multiple sclerosis (Copaxone®, Teva Pharm; $3.7 billion), and the polymer conjugate Pegfilgrastim for the treatment of neutropenia (Neulasta®, Amgen; $3.6 billion). Poly(ethylene glycol) (PEG) is still the most frequently used polymer so far and also the gold standard for stealth polymers in the emerging field of polymer based drug delivery. The increasing use of PEG and PEGylated products in pharmaceutical research and on the market not only provides new insight into the underlying mechanism of the beneficial properties of PEG, it also increased the likelihood of encountering potentially unfavorable effects.
These can be divided into several groups:
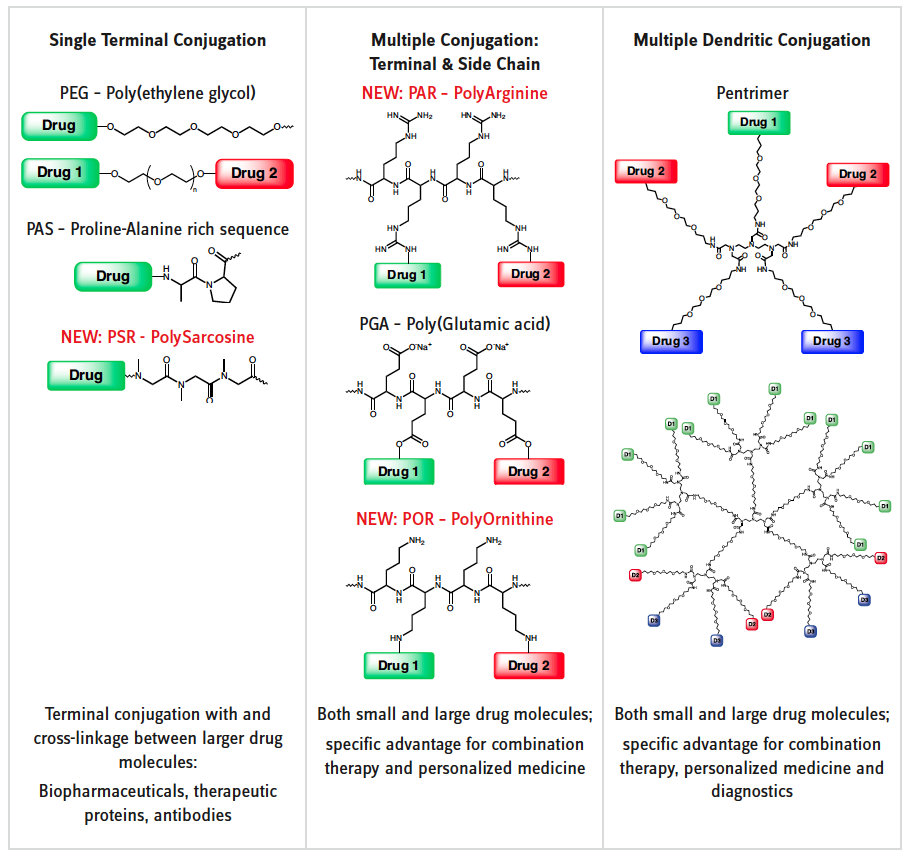
In particular the non-biodegradability of PEG led to the fact that plenty of new carriers have been developed. Most of them are based on poly(amino acids). The repeating functional group is an amide bond, just like in any natural peptide and protein. Proteases thus can hydrolyze the polymer and degrade the whole conjugate. Larger molecular weights and higher doses for application are possible. Each polymer carrier technology has its specific characteristics and strong points for certain application fields (see scheme on left page).
PEG, PAS and PSR are linear polymers. Conjugation is only possible on the termini. Terefore, these types of carriers are mostly conjugated with larger molecules like antibodies or therapeutic proteins. PAS and PSR overcome the major drawback of PEG, i.e. its non-biodegradability. PAS is a recombinantly produced polymer, therefore strictly monodisperse. The advantages are excellent biodegradability and good analytics. The drawback is that application is limited to recombinant production of biopharmaceuticals only. Chemical conjugation is possible, however, only to a very limited extent.
PSRs are polydisperse just like PEG. They are peptoids and can therefore be degraded by proteases. Higher molecular weights and higher doses can be applied. A large variety of PSRs with different functional groups are readily available and others can easily be designed.
Poly(amino acids) of monomers with reactive side chains open the field of polymer therapeutic also to small molecules which can be conjugated to the polymer backbone through both terminal and plenty of side chain conjugations. Multiple loading can be achieved and also loading with several and different drug compounds or analytical or therapeutic agents. Combination therapy, personalized medicine and diagnostics are applications that are very easily accessible through these new carriers.
A fascinating concept are Pentrimers which can carry up to three different conjugates on the same pentavalent center molecule of a first generation dendrimer. Higher generations offer multiplex conjugations for sophisticated applications in therapy and diagnostics.
References: