Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 09/02/2017
3.7.2 Mercapto-PEG-Acids
S-Acetyl-PEG-acids and active esters provide a method for converting amino groups to a thiol, while incorporating PEG units. Conjugation with thiol reactive agents e.g., maleimides, vinyl sulfones or α-halo keto functionalized reaction partners increases the conjugation possibilities of the former amino group by the whole set of thiol reactive reagents. Mercapto-PEG-Acids are highly hydrophilic, non-antigenic, non-immunogenic and non-toxic.
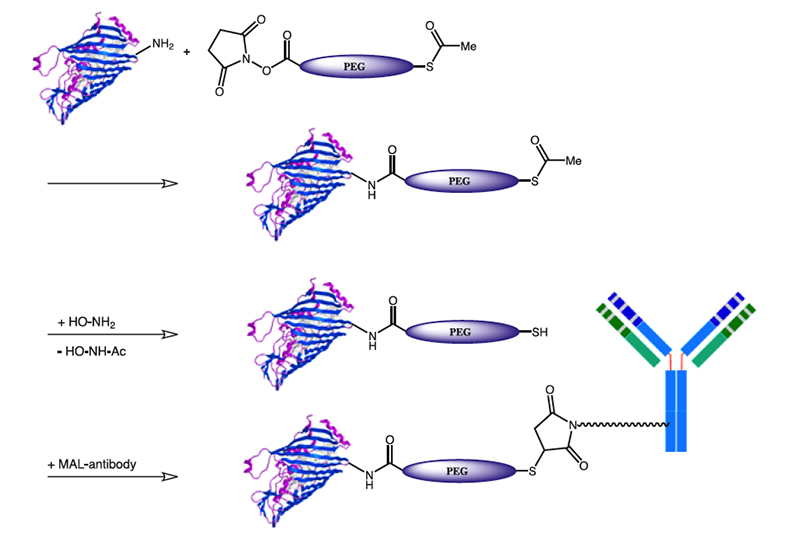
Protocol for in-situ Activation of PEG-Acids to the NHS ester:
Add a methylene chloride solution of the acid to the dry reagents under dry conditions (10-20% molar excess of EDC and NHS in dry methylene chloride, dried over 3A molecular sieves). Stir for several hours or overnight, then evaporate the solvent and use. The reaction mixture can also be treated with a small amount of silica gel to adsorb the excess EDC and the urea by-product. Filter, then evaporate the solvent and use. NHS should be added together with EDC to prevent formation of the anhydride. DCC and DIC can also be used. Typically use about 1 equivalent, and add a solution of the carbodiimide to the acid and NHS (1.1 to 1.2 eq.). PfOH (pentafluorophenol), MSNT (1-(Mesitylene-2-sulfonyl)-3- nitro-1,2,4-triazole), HOCt (Ethyl 1-hydroxy-1H-1,2,3-Triazole- 4-carboxylate), HOPO (2-Hydroxypyridine-N-oxide) and a set of other coupling reagents/leaving groups can be used in place of NHS, if this is of any preference.
Reference and Protocols:
OPSS Protected Mercapto-PEG Crosslinkers
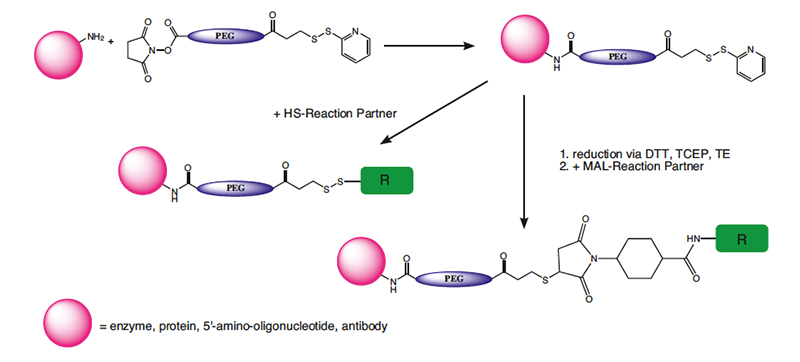
The general application of OPSS protected mercapto-PEG crosslinkers is the controlled and selective conjugation of an amine containing target, which reacts first with the NHS ester and then subsequently with a sulfhydryl containing complementary target molecule to form another disulfide. Many biological molecules contain both the amine function and the complementary compounds. Peptides, oligonucleotides or other biologicals, for example, can be terminated with thiols or have thiols designed into them, and vice versa. The contrast with the maleimide containing crosslinkers is that the OPSS derivatives form a disulfide, stable under non-reducing conditions. Normally, thiols can be cleaved with a reducing agent or exchanged with another thiol. The OPSS group presents the potential of using the pyridine- 2-thione, released in the reaction with another thiol, to measure the level of the PEG-OPSS incorporation in the labeling step or to monitor the subsequent reaction with another thiol by measuring its absorption at 343 nm. The cleavable OPSS PEGylating reagents produce a disulfide bond with thiols, which can later be cleaved with a variety of reducing agents like DTT or TCEP, or react with another thiol.
References: