Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 09/02/2017
b) Peptide Synthesis with Azido and Alkyne Amino Acids
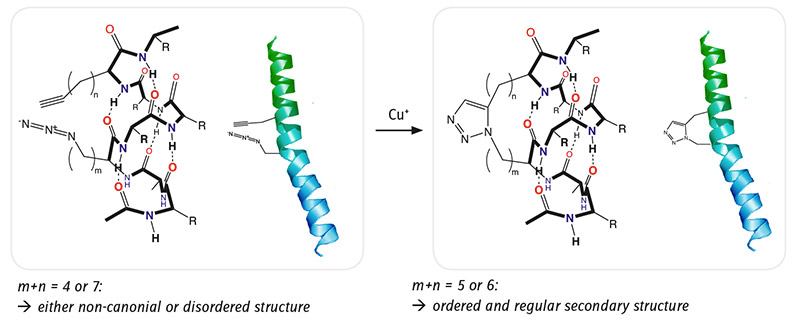
As Boc and Fmoc protected derivatives of both azido and alkyne amino acids are available, they can be introduced into peptide sequences through standard SPPS protocols, for example. In an α-helical secondary structure amino acids at positions i and i+4 are above each other. When they carry alkyne and azido function respectively, these groups are close to each other and can undergo cyclo-addition forming the corresponding 1,2,3-triazol moiety and stabilizing the secondary structure in the α-helical conformation. It has been demonstrated with nonapeptides [1,2] that whenever the 1,2,3-triazol bridge carries 5 or 6 methylene groups, the peptide shows a nice regular and ordered secondary structure. However, if the ring size is smaller (m+n= 4) or larger (m+n=7) the secondary structure is rather disordered.
Stabilization of the secondary structure also can be achieved by side chain lactam formation, for example with Lys and Asp at the appropriate positions. Azido and alkyne amino acids have, however, from synthetic and cost points of view some advantages. Using Lys and Asp orthogonal protecting groups have to be selected, which can result in a more complex process and more expensive building blocks. Azido and alkyne functions react highly specific to the 1,2,3-triazol ring. Educts and products are stable and inert at normal peptide synthesis conditions. No additional protection and related deprotection steps are required and the 1,2,3-triazol shows proteolytic stability, which is of high importance, whenever built into biological or pharmacological products.
Protocol for click reaction in peptide synthesis: Successful protocols have been published applying to 3 µmol peptide in 4 ml tBuOH/H2O (1:2) with excess of ascorbic acid (40 µmol) and CuSO4*5H2O (40 µmol) generating Cu(I) in situ. Stirring at room temperature over night is followed by appropriate chromatographic work up. [3]
References: