Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 09/12/2014
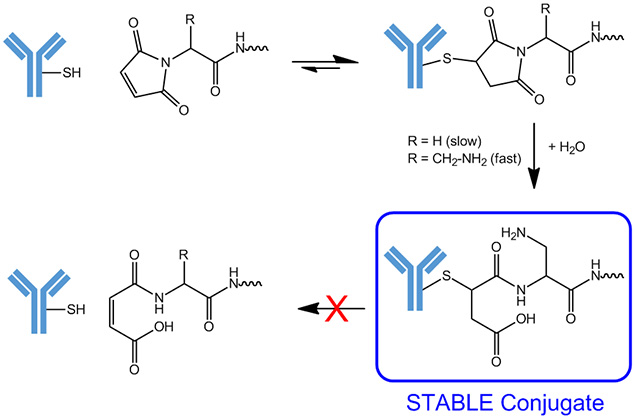
Maleimide (MAL) reacts rather specific with free thiol groups and forms appropriate conjugates. However, this reaction is reversible, resulting in loss of the conjugation partner.
Amino functions open the maleimido ring structure through hydrolysis with nucleophilc neighboring effect and thus inhibit the undesired release reaction.
Therefore, our MAL-Dap(Boc) building blocks are ideal tools for persistent conjugation with thiol groups: After removal of the Boc protecting group and conjugation with the mercaptane function, the free methylamino side chain will open the maleimide ring under aqueous conditions. While the conjugation reaction between thiol and cyclic maleimide is reversible, it turns completely stable in the open ring structure. This becomes particularly important in the formation of conjugates with high value components, like in antibody-drug conjugation (ADC).
We supply the appropriate MAL building blocks above promptly from stock and any other MAL conjugate on custom synthesis basis.
