Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 05/03/2024

The cyclic amino acid proline (Pro, P) represents unique features in terms of its conformational properties. This is even more true for cis-4-aminoprolines (cis-4-Amp): the amino group and the carboxylic acid are in proximity and possess a clamped stereochemistry, as they are attached to the circular, strained pyrrolidine system. The result is an intramolecular hydrogen bond which can exert a strong influence on the tertiary structure of the final peptide, stabilizing beta- and gamma-turns, respectively.
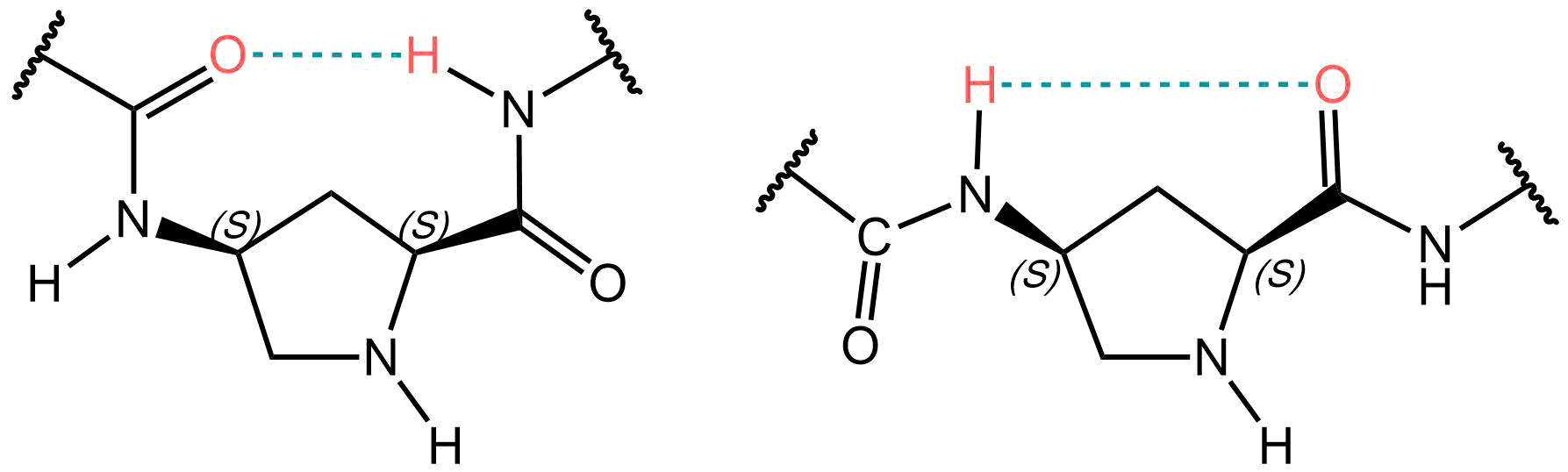
Two possibilities for hydrogen bond formation in cis-4-aminoproline.
Thus, cis 4-Amp can be used to induce both beta- and gamma-turns, depending on the positioning of the intramolecular hydrogen bond between the amino- and the carboxyl group. The gamma-turns play a role in initiating the formation of beta-sheets during protein folding. Beta- and gamma turns are also recognized as important sites for posttranslational modifications, particularly for phosphorylation and glycosylation. Moreover, replacing a native proline with 4-Amp can lead to significantly improved water solubility, due to the added polar amino group. Another fascinating application of 4-Amps involves building cyclic peptides around it and utilizing the amino group at position 4 as an anchor, i.e., by connecting it to a scaffold or carrier molecule.
The unique steric and stereoelectronic characteristics of amino and ammonium groups can be harnessed to create pH-responsive probes: For instance, in (2S,4S)-4-Amp, the configuration shifts from exo (chair) in alkaline conditions to endo (boat) in acidic pH. This change is supported by the formation of a transannular hydrogen bond when the primary amino group is protonated and generates a remarkable feature which enables the regulation of pH-dependent denaturation in peptides and peptide assemblies, such as collagens. A possible application of this effect is the controlled release of payloads like medications or effector molecules.
To introduce 4-Amp by solid-phase peptide synthesis, the amino groups require orthogonal protection, e.g. Boc and Fmoc. At Iris Biotech, various suitable derivatives are available (see related products).

Two examples for orthogonally protected amino-groups in 4-Amp.
|
Iris product |
Body |
Conformation |
Protection at the |
Protection at the |
|
FAA3205 |
L-Proline |
2S, 4R |
Fmoc |
Boc |
|
FAA3210 |
L-Proline |
2S, 4S |
Fmoc |
Boc |
|
FAA4640 |
L-Proline |
2S, 4R |
Fmoc |
Alloc |
|
FAA3175 |
L-Proline |
2S, 4S |
Fmoc |
Alloc |
|
FAA7130 |
L-Proline |
2S, 4S |
Fmoc |
Poc |
|
BAA1783 |
L-Proline |
2S, 4R |
Boc |
Fmoc |
|
FAA3240 |
L-Proline |
2S, 4S |
Boc |
Fmoc |
|
BAA3690 |
D-Proline |
2R, 4R |
Boc |
Fmoc |
|
BAA4570 |
D-Proline |
2R, 4S |
Boc |
Fmoc |
Abbreviations: Fmoc = Fluorenylmethyloxycarbonyl; Boc = t-Butyloxycarbonyl; Alloc = Allyloxycarbonyl; Poc = Propargyloxycarbonyl.
→ You’re looking for a different Aminoproline not listed in our catalog? Get in contact for a custom synthesis!
→ More Proline- and Pseudoproline molecules are summarized in our (Pseudo-)Prolines Flyer.
References:
N-α-benzoyl-cis-4-amino-L-proline: A γ-turn mimetic; T. P. Curran, N. M. Chandler, R. J. Kennedy, M. T. Keaney; Tetrahedron Letters 1996; 37: 1933-1936. https://doi.org/10.1016/0040-4039(96)00307-3
Total Synthesis and Antifungal Evaluation of Cyclic Aminohexapeptides; L. L. Klein, L. Li, H.-J. Chen, C. B. Curty, D. A. DeGoey, D. J. Grampovnik, C. L. Leone, S. A. Thomas, C. M. Yeung, K. W. Funk, V. Kishore, E. O. Lundell, D. Wodka, J. A. Meulbroek, J. D. Alder, A. M. Nilius, P. A. Lartey, J. J. Plattner; Bioorganic & Medicinal Chemistry 2000; 8: 1677-1696. https://doi.org/10.1016/S0968-0896(00)00097-3
The cis-4-Amino-L-proline Residue as a Scaffold for the Synthesis of Cyclic and Linear Endomorphin-2 Analogues; A. Mollica, F. Pinnen, A. Stefanucci, F. Feliciani, C. Campestre, L. Mannina, A. P. Sobolev, G. Lucente, P. Davis, J. Lai, S.-W. Ma, F. Porreca, V. J. Hruby; J. Med. Chem. 2012; 55: 3027-3035. https://doi.org/10.1021/jm201402v
cis-4-Amino-l-proline Residue as a Scaffold for the Synthesis of Cyclic and Linear Endomorphin-2 Analogues: Part 2; A. Mollica, F. Pinnen, A. Stefanucci, L. Mannina, A. P. Sobolev, G. Lucente, P. Davis, J. Lai, S.-W. Ma, F. Porreca, V. J. Hruby; J. Med. Chem. 2012; 55: 8477–8482. https://doi.org/10.1021/jm300947s
pH-Responsive Aminoproline-Containing Collagen Triple Helices; J. Egli, C. Siebler, B. Maryasin, R. S. Erdmann, C. Bergande, C. Ochsenfeld, H. Wennemers; Chemistry 2017; 23: 7938-44. https://doi.org/10.1002/chem.201701134
Application of (4R)-aminoproline in peptide engineering: conformational bias and pH-responsiveness revisited; V. Kubyshkin; New J Chem. 2022; 46: 9587-9594. https://doi.org/10.1039/D2NJ00305H
New 4-Aminoproline-Based Small Molecule Cyclopeptidomimetics as Potential Modulators of α4β1 Integrin; A. Sartori, K. Bugatti, E. Portioli, M. Baiula, I. Casamassima, A. Bruno, F. Bianchini, C. Curti, F. Zanardi, L. Battistini; Molecules 2021; 26: 6066-6087. https://doi.org/10.3390/molecules26196066