Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 21/02/2022
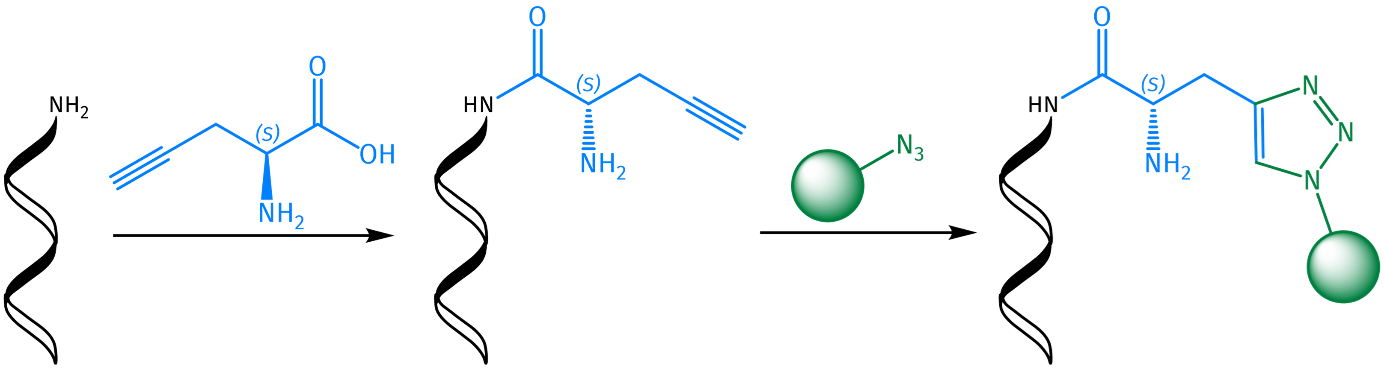
Alkynes and azides can undergo a Cu(I)-catalyzed azide alkyne 1,3-dipolar cycloaddition (CuAAC) to afford 1,4-disubstituted 1,2,3-triazoles. Developed by K. Barry Sharpless and Morten Meldal, this type of chemical transformation was quickly dubbed “Click Chemistry”. It has since become a widely used reaction that is orthogonal to many other types of chemical transformations and is used in various kinds of applications. Due to its high thermodynamic driving force, which is usually greater than 20 kcal/mol, the Click reaction rapidly proceeds to completion in almost all cases. Moreover, while the thermal Huisgen 1,3-dipolar cycloaddition affords a mixture of both the 1,4-substituted and the 1,5-substituted regioisomers, the CuAAC is highly selective for the 1,4-substituted isomer only.
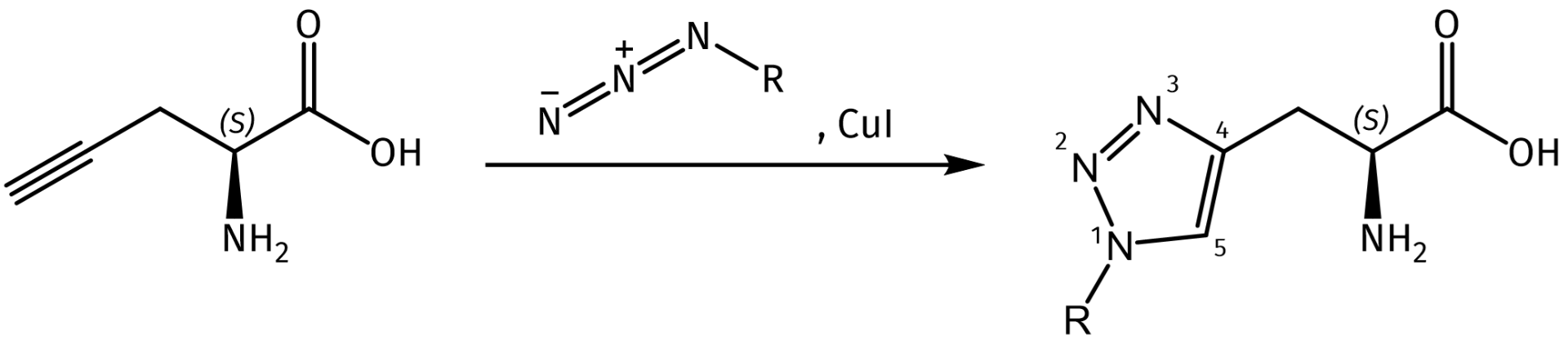
The copper-catalyzed azide-alkyne cycloaddition affording the 1,4-disubstituted isomer.
Among scientists, CuAAC has found widespread use as a biochemical tool as it represents a convenient method to chemoselectively functionalize peptides at specific positions without having to resort to complicated protecting group chemistry. Furthermore, Click chemistry facilitates otherwise challenging reactions such as peptide macrocyclization.
Iris Biotech offers an extensive selection of different propargyl amino acids for both Fmoc and Boc strategies, as well as unprotected propargyl amino acids for enzymatic peptide synthesis. The presence of an azido or alkyne function at a particular position of a peptide sequence opens up the possibility for the site-selective conjugation of other biomolecules, labels, or APIs. By varying the amino acid bearing the propargyl moiety, different points of conjugation within the peptide chain can be evaluated to find the best suitable one for modification to achieve the best result without affecting the properties of the native peptide.
Besides, Iris Biotech is offering a variety of azide-functionalized amino acids.
If you are looking for metal catalyst-free ways of Click-chemistry, Iris Biotech provides a variety of DBCO, DACN, and tetrazine derivatives for inverse electron-demand Diels-Alder (IEDDA) reactions and strain-promoted azide-alkyne cycloadditions (SPAACs).
In addition, Iris Biotech’s portfolio includes Tris(benzyltriazolylmethyl)amine (TBTA; RL-2010), which is stabilizing copper(I) towards oxidation in solution by forming a complex. Thus, it effectively catalyzes quantitative and regioselective Click cycloaddition reactions in a variety of aqueous and organic solvents.
THPTA (RL-2210) is a water-soluble alternative to TBTA and a highly efficient ligand for Click chemistry in partially organic and particularly in completely aqueous reactions. The benefits of a completely aqueous reaction include the biological labelling of live cells or the labelling of proteins without the concern of denaturing secondary structures. THPTA complexes Cu(I) and thus blocks its bioavailability. This mitigates potentially toxic effects while maintaining the catalytic effectiveness in Click conjugations.
➔ Interested in Click Chemistry? Download our Brochure to get detailed information and product overview!
References:
Expanding the Genetic Code of Yeast for Incorporation of Diverse Unnatural Amino Acids via a Pyrrolysyl-tRNA Synthetase/tRNA Pair; S. M. Hancock; R. Uprety, A. Deiters, J. W. Chin; J. Am. Chem. Soc. 2010; 132(42): 14819-14824. https://doi.org/10.1021/ja104609m.
Reprogramming Nonribosomal Peptide Synthetases for “Clickable” Amino Acids; H. Kries, R. Wachtel, A. Pabst, B. Wanner, D. Niquille, D. Hilvert; Angew. Chem. Int. Ed. 2014; 53(38): 10105-10108. https://doi.org/10.1002/anie.201405281.
Click Chemistry as a Macrocyclization Tool in the Solid-Phase Synthesis of Small Cyclic Peptides; R. A. Turner, A. G. Oliver, R. S. Lokey; Org. Lett. 2007; 9(24): 5011-5014. https://doi.org/10.1021/ol702228u.
Specific and quantitative labeling of biomolecules using click chemistry; K. Horisawa; Front. Physiol. 2014; (5). https://doi.org/10.3389/fphys.2014.00457.