Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 27/04/2020
The original definition of "bioisostere" as introduced by Harris Friedman in 1950 described compounds eliciting similar biological effects. More recently, this definition of bioisosteres was broadened by Burger to encompass "compounds or groups that possess near-equal molecular shapes and volumes, approximately the same distribution of electrons, and which exhibit similar physical properties". (Prog. Drug Res. 1991, 37, 287.)
The development and application of bioisosteres is nowadays considered standard practice in medicinal chemistry. This fundamental tactical approach is used to address various aspects associated with the design and development of drug candidates, such as improving potency, enhancing selectivity, altering physical properties, reducing or redirecting metabolism, eliminating or modifying toxicophores, or acquiring novel intellectual property.
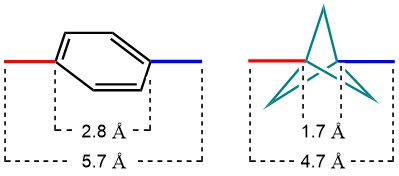
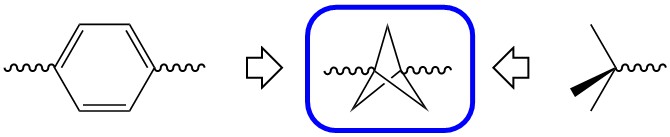
The use of multiple phenyl groups in APIs is connected with various downsides, for example their rapid metabolic breakdown, resulting in low bioavailability and low plasma concentrations. Moreover, low aqueous solubility, too high lipophilicity, CYP450 inhibition and hERG inhibition are potential pitfalls of a high aromatic ring count. The bicyclo[1.1.1]pentane (BCP) motif is a nonclassical phenyl ring bioisostere. The rationale for its use is illustrated in the figure below. When comparing a 1,4-substituted phenyl ring with a comparably substituted BCP, not only are the dihedral angles between substituents identical, also the distances between the substituents are very similar.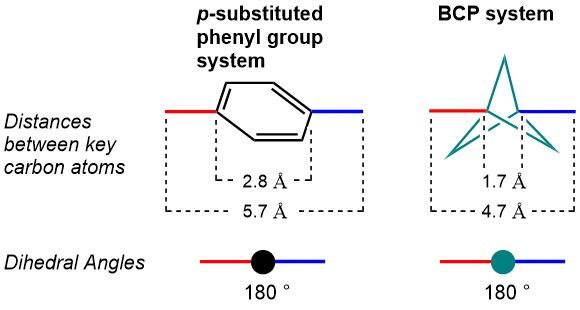
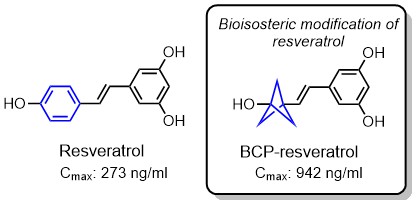
The usefulness of bicyclo[1.1.1]pentane was successfully demonstrated in the case of Resveratrol. The biological significance of Resveratrol is well-established, but so are the issues related to bioavailability of this potentially useful natural product. BCP-resveratrol, however, shows significant improvement in the bioavailability and shows improved half-life and intrinsic clearance. Metabolite formation, like glucuronide and sulfate conjugation is drastically reduced.
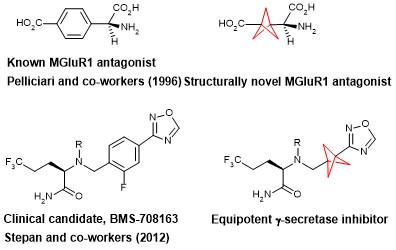
The BCP motif is already being used in the development of bioactive molecules and its biocompatibility has been proven by in vivo pharmacokinetics (liver and kidney profiling and efficacy studies) in several cases.
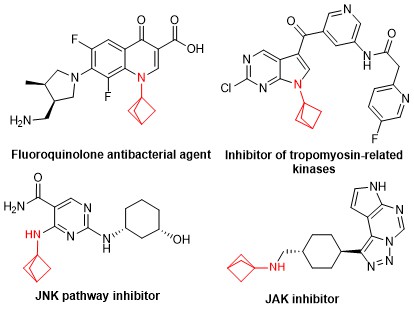
Moreover, it is a diversifiable motif, offering a multitude of functionalizations based on the BCP core. Routes of synthesis are metal free, efficient and scalable, and thus suitable for commercial applications. Besides replacing phenyl groups, BCP is also a good bioisostere for tert-butyl groups.
➔ Visit our Webshop to discover our selection of amino acids carrying BCP and other bicyclic motifs.
➔ Do you require a custom-made BCP derivative? Send us your inquiry!
References: