Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 09/02/2017
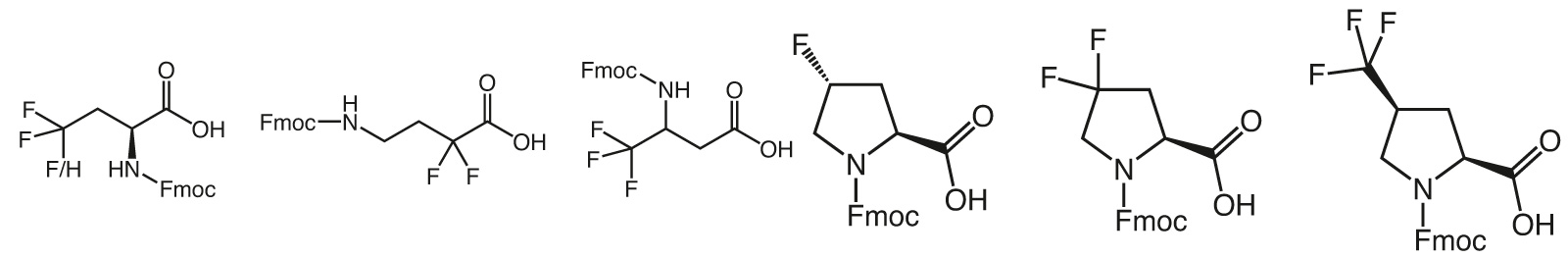
The incorporation of trifluoromethyl group carrying building blocks into peptides results in increased chemical and thermal stability, increased resistance to degradation by proteases, and enhanced lipophilicity. Trifluoromethyl group bearing peptides will therefore show a better affinity to lipid membranes and stronger interactions with receptors.
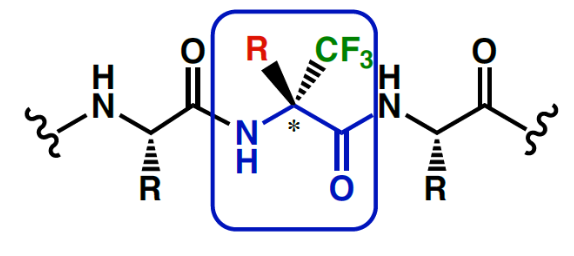
However, the high electronegativity of Fluorine has drastic effects on the synthetic routes of any fluorine containing molecule. Hence, production of trifluormethyl or other fluoro derivatives require specific know- how and experience and the resulting building blocks display unique properties.
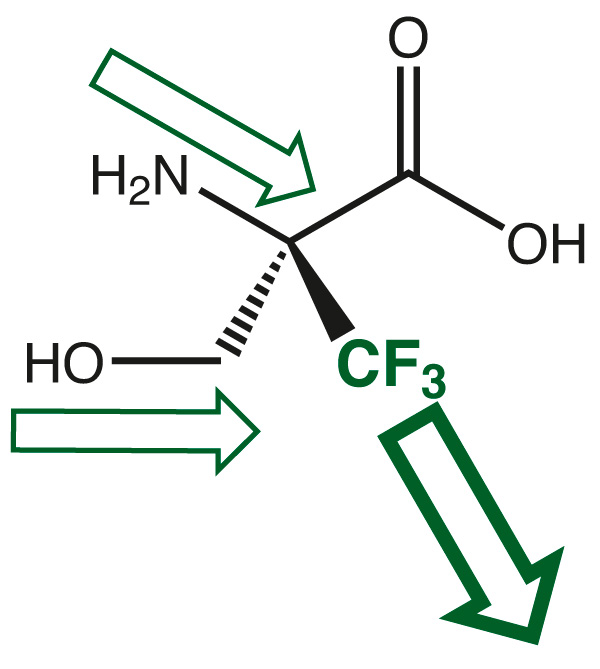
The electron withdrawing effect of the trifluoromethyl group leads to high electron deficiency at the neighboring amino function and in the case of serine also at the hydroxy group. Both nitrogen and oxygen suffer their nucleophilicity and require not anymore any protection during any standard electrophilic coupling procedures during peptide synthesis.
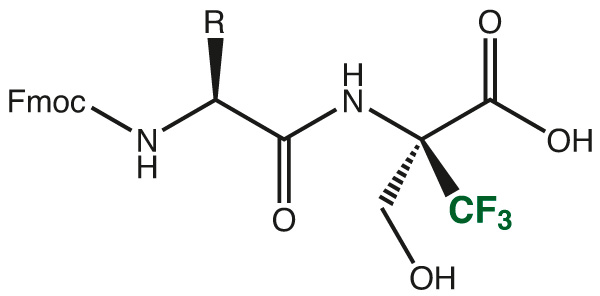
An easy and convenient way, therefore is the use of protected dipeptide fragments, which easily can be incorporated in standard Fmoc/tBu synthesis protocols in automated or manual SPPS.
➔ Please inquire for the Trifluoromethyl-dipeptide of your choice.