Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 06/02/2024

The chemical alteration of proteins by post-translational modifications (PTMs) plays an important role in diverse cellular and pathological processes. Lysine malonylation is a recently characterized PTM involved in various metabolic processes and stress responses. Besides, malonylation modifications are closely related to the pathogenesis of various diseases, such as inflammation, type 2 diabetes, and angiogenesis-associated diseases.
One drawback of the before-mentioned modification is its potential susceptibility to decarboxylation, making it difficult to analyze the original malonyllysine and causing artifactual detection of acetyllysine at former malonylation sites. Hence, studies of post-translational modifications greatly benefit from the development of stable analogues of these modifications, allowing for their incorporation in proteins and functional analysis.
To address this, we report on a stable isostere of malonyllysine. Replacement of the malonyl group by a tetrazole results in resistance towards thermal decarboxylation, while keeping similar physical properties such as a planar structure and nearly identical pKa values (carboxylate pKa 4.5; tetrazole pKa 4.9).
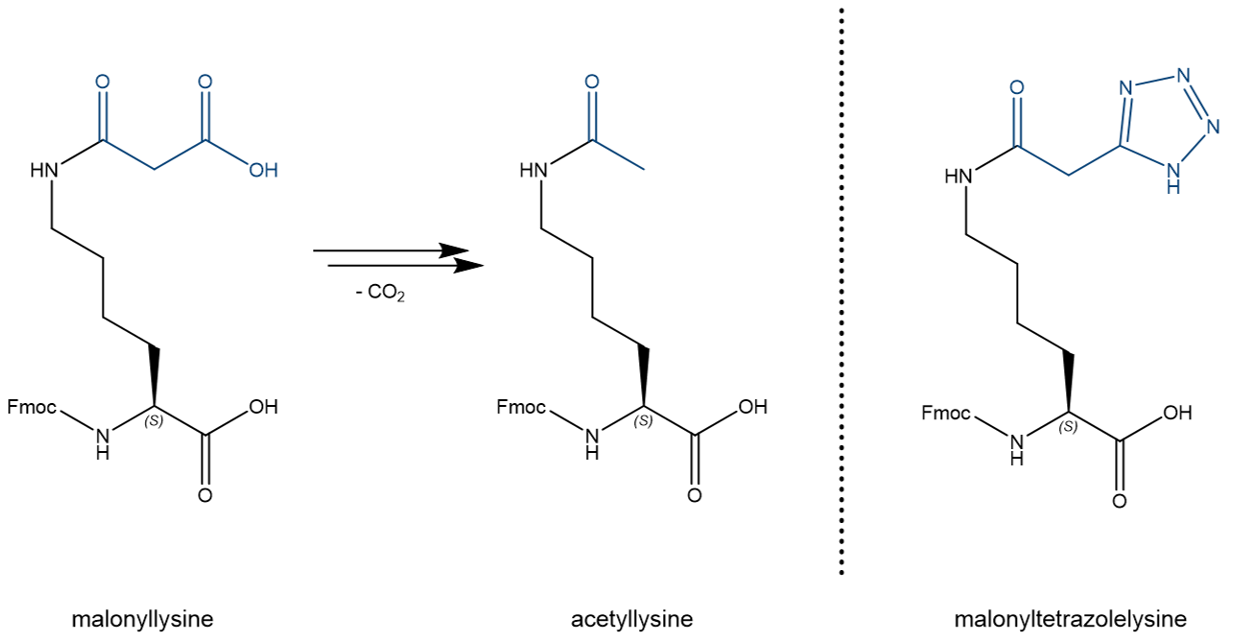
Malonyllysine suffers from decarboxylation resulting in the formation of acetyllysine. Malonyltetrazolelysine is a thermally and chemically stable isostere, which is available as Fmoc protected derivative, hence, can be incorporated into peptide sequences by standard synthetic protocols.
At Iris Biotech, we offer a Fmoc-protected derivative suitable for incorporation into a peptide by solid-phase peptide synthesis (SPPS). This compound is ideally for the analysis of post-translational lysine derivatizations. Besides, other protecting groups can be installed enabling the synthesis of all kinds of peptide substrates, chemical attachment to proteins, conjugation to antibodies, single-chain and nanobodies via incorporation of non-canonical amino acids and Click reaction, and to non-biological carriers, such as polymers, silicates, metal surfaces or metal oxides.
Besides its superior thermal and chemical stability, malonyltetrazolelysine is also recognized by anti-malonyllysine antibodies and SIRT5 decarboxylases with kinetics comparable to the original malonyllysine. Furthermore, UV absorption of the tetrazole derivative enables spectroscopic monitoring by a set of different analytical methods, which allows more accurate analysis than the endogeneous malonyl derivative.
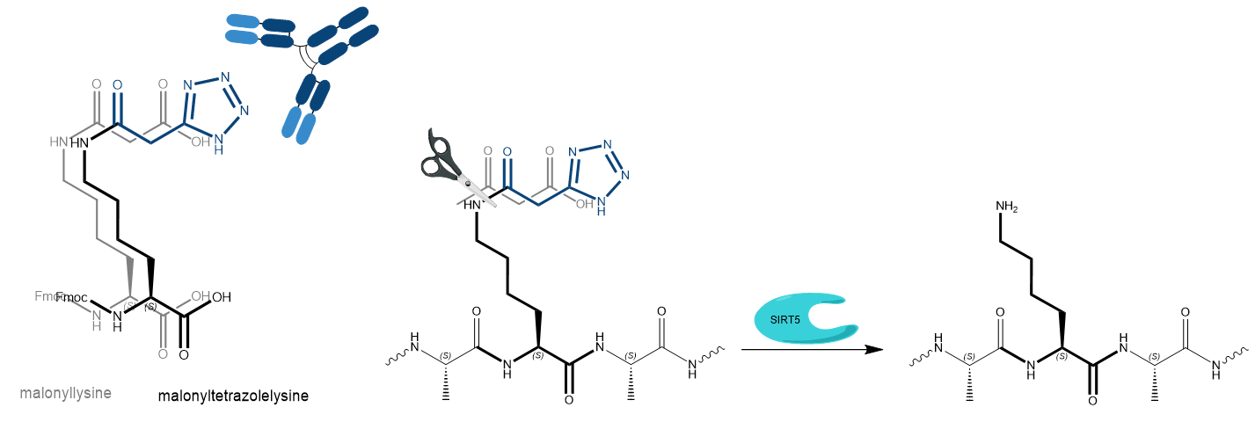
The tetrazole isostere of malonyllysine is recognized by anti-malonyllysine and histone deacetylases. SIRT5 removes malonyl and malonyltetrazole, respectively, with comparable kinetics.
Summarized properties of malonyltetrazolelysine:
→ You are interested in another protection pattern? Get in contact and inquire for a custom synthesis!
→ Interested in anti-malonyl antibodies decorated with payloads via stable or self-immolative linkers?
References:
Synthesis and Evaluation of a Stable Isostere of Malonyllysine**; Y. Jing, S. E. Bergholtz, A. Omole, R. A. Kulkarni, T. T. Zengeya, E. Yoo, J. L. Meier; Chembiochem : a European journal of chemical biology 2022; 23: e202100491. https://doi.org/https://doi.org/10.1002/cbic.202100491
Lysine Malonylation and Its Links to Metabolism and Diseases; L. Zou, Y. Yang, Z. Wang, X. Fu, X. He, J. Song, T. Li, H. Ma, T. Yu; Aging Dis. 2023; 14(1): 84-98. https://doi.org/10.14336/AD.2022.0711
Chapter 1 – Posttranslational Modifications of Proteins and Their Role in Biological Processes and Associated Diseases; I.-u.-R. Tak, F. Ali, J. S. Dar, A. R. Magray, B. A. Ganai, M. Z. Chishti; Protein Modificomics 2019; 1-35. https://doi.org/10.1016/B978-0-12-811913-6.00001-1