Two selected publications were summarized in this article in order to highlight the impressive utility of amino acids incorporating the diazirine photophore.
Iris Biotech offers a comprehensive set of diazirine-functionalized amino acids for photo-crosslinking experiments. Upon irradiation with UV light (ca. 350 nm - 360 nm), the diazirine moiety yields a highly reactive carbene species that can undergo insertions into C-C, C H, O-H and X-H (X = heteroatom) bonds of neighboring molecules, forming a covalent bond. The diazirine moiety is the smallest of all photophores, so introduction of a diazirine-bearing amino acid into a peptide or protein usually does not impair its biological activity. Further advantages of diazirine crosslinkers are their stability at room temperature, as well as their relative stability to nucleophiles, and to both acidic and basic conditions.
Two recent examples of the application of diazirine amino acids are highlighted below.
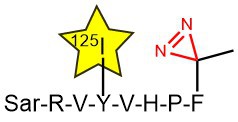
In one published experiment, Fmoc-L-Photo-Phe-OH (FAA5690) was incorporated into an angiotensin-II-derived peptide probe in place of the native Phe8. By irradiating the AngII type 2 receptor-bound octapeptide with UV light, the precise ligand binding site on the receptor could be determined (D. Fillion
et. al. 2006,
J. Med. Chem.).
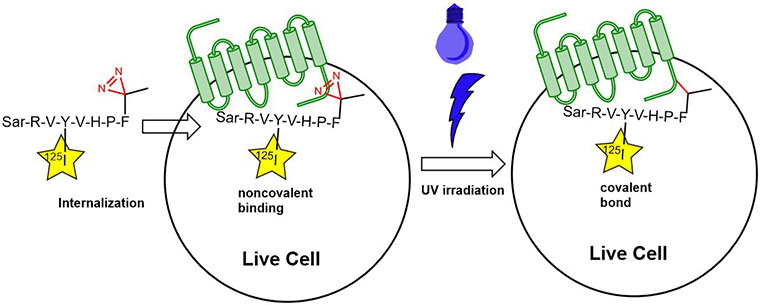
Use of Photo-Phenylalanine for identification of Angiotensin-II receptor binding sites; 125I is used as a
radiotracer.
Another publication describes the incorporation of Photo-Phe into a potential aggregation inhibitor of amyloid-β (Aβ). The known Aβ binder cyclo-KLVFF was modified to afford diazirine-functionalized cyclo-KLVF(β-Ph)-Tdf (Tdf = 4-(
trifluoromethyl
diazirin)-L-
phenylalanine). Upon binding to amyloid-β, UV-irradiation leads to the formation of a covalent bond between inhibitor and target. It was shown that this modification decreased the tendency of Aβ to form cross-β-sheet structures, as well as the toxicity of Aβ1–42 (R. Kino
et. al. 2015,
Bioorg. Med. Chem. Lett.).
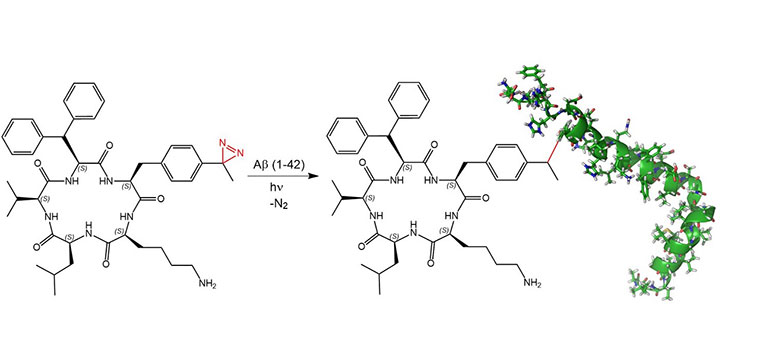
Covalent modification of Aβ1–42 with cyclo-KLVF(β-Ph)-Tdf.
Get our free Photochemistry booklet for a comprehensive overview of our photoactivated compounds.
References:
- Stereospecific Synthesis of a Carbene-Generating Angiotensin II Analogue for Comparative Photoaffinity Labeling: Improved Incorporation and Absence of Methionine Selectivity; D. Fillion, M. Deraët, B. J. Holleran and E. Escher; Journal of Medicinal Chemistry 2006; 49: 2200-2209. doi:10.1021/jm050958a
- Covalent modifier-type aggregation inhibitor of amyloid-β based on a cyclo-KLVFF motif; R. Kino, T. Araya, T. Arai, Y. Sohma and M. Kanai; Bioorganic & Medicinal Chemistry Letters 2015; 25: 2972-2975. doi:https://doi.org/10.1016/j.bmcl.2015.05.027