Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 02/02/2015
2,4-Dimethylbenzyl (Dmb) or 2,4,6-Trimethylbenzyl (Tmb) can be used as auxiliary protecting groups for temporarily masking the amide nitrogen of a peptide bond. Furthermore, Dmb and Tmb amino acids prevent aggregation during solid phase synthesis, prevent aspartimide formation when introduced before an Asp residue and disrupt aggregation like pseudoprolines. They can also increase peptide cyclization efficiency.
For ease of synthesis, new dipeptide building blocks can be used to couple two amino acids with one process. Acidic deprotection methods as normally applied on Wang resin finally generate the native sequence.
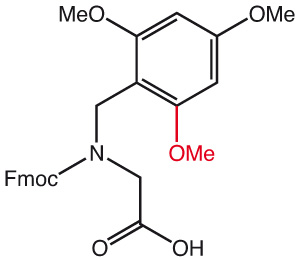
Standard coupling protocols can be applied with reagents such as PyBOP, DIC/HOBt or HATU. Acidic deprotection methods normally used with Wang resin finally generate the native sequence.